the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Dipolar order mediated 1H → 13C cross-polarization for dissolution-dynamic nuclear polarization
Stuart J. Elliott
Samuel F. Cousin
Quentin Chappuis
Olivier Cala
Morgan Ceillier
Aurélien Bornet
Sami Jannin
Magnetic resonance imaging and spectroscopy often suffer from a low intrinsic sensitivity, which can in some cases be circumvented by the use of hyperpolarization techniques. Dissolution-dynamic nuclear polarization offers a way of hyperpolarizing 13C spins in small molecules, enhancing their sensitivity by up to 4 orders of magnitude. This is usually performed by direct 13C polarization, which is straightforward but often takes more than an hour. Alternatively, indirect 1H polarization followed by 1H→13C polarization transfer can be implemented, which is more efficient and faster but is technically very challenging and hardly implemented in practice. Here we propose to remove the main roadblocks of the 1H→13C polarization transfer process by using alternative schemes with the following: (i) less rf (radiofrequency) power; (ii) less overall rf energy; (iii) simple rf-pulse shapes; and (iv) no synchronized 1H and 13C rf irradiation. An experimental demonstration of such a simple 1H→13C polarization transfer technique is presented for the case of [1-13C]sodium acetate, and is compared with the most sophisticated cross-polarization schemes. A polarization transfer efficiency of ∼0.43 with respect to cross-polarization was realized, which resulted in a 13C polarization of ∼8.7 % after ∼10 min of microwave irradiation and a single polarization transfer step.
- Article
(1148 KB) - Full-text XML
-
Supplement
(259 KB) - BibTeX
- EndNote
Traditional magnetic resonance imaging (MRI) and spectroscopy (MRS) experiments usually suffer from low sensitivity. Hyperpolarization techniques including dissolution-dynamic nuclear polarization (dDNP) can be used to highly polarize a large variety of chemical systems and therefore enhance nuclear magnetic resonance (NMR) signals by several orders of magnitude (Ardenkjær-Larsen et al., 2003). Various applications of dDNP have been demonstrated, including the study of enzyme kinetics, cell extracts and heteronuclear metabolomics (Bornet et al., 2014b, 2016a; Dumez et al., 2015). Most dDNP applications involve the use of weakly magnetic isotopes such as 13C, but excessively long DNP timescales τDNP(13C) hinder efficient polarization build-up and lead to extended experimental times. Intrinsically sensitive proton nuclear spins do not suffer from such issues and can be polarized quickly and to a greater extent at low temperatures (Hartmann et al., 1973).
The use and optimization of cross-polarization (CP) under dDNP conditions (typically at temperatures of about 1.2–1.6 K in superfluid helium) provides a way to substantially boost 13C polarizations and enhance build-up rates 1∕τDNP(13C) (by a factor of up to 40) (Hartmann and Hahn, 1962; Pines et al., 1972; Perez Linde, 2009; Jannin et al., 2011; Bornet et al., 2012, 2013; Batel et al., 2012; Vuichoud et al., 2016; Cavaillès et al., 2018). The technique requires intense B1 matching (typically > 15 kHz) of simultaneous 1H and 13C spin-locking radiofrequency (rf) fields throughout an optimized contact period (typically > 1 ms). This CP-DNP approach recently turned out to be key for the preparation of transportable hyperpolarization (Ji et al., 2017), where samples are polarized in a CP-equipped polarizer and then transported over extended periods (typically hours or days) to the point of use.
This CP approach was demonstrated on typical dDNP samples back in 2012 (Bornet et al., 2012); however, the technique remains challenging today because of its methodological and technical complexity. Indeed, CP under dDNP conditions employs sophisticated pulse sequences, and involves high-power and high-energy rf pulses. Another drawback of CP-DNP is that it can hardly be scaled up to volumes larger than 500 µL, otherwise engendering detrimental arcing in the superfluid helium bath (Vinther et al., 2019). Such scaling-up would be required to enable parallel hyperpolarization of multiple transportable samples (Lipsø et al., 2017), and for volumes > 1 mL currently used for hyperpolarized human imaging (Nelson et al., 2013).
For hyperpolarizing larger sample volumes, alternative rf sequences with reduced power requirements are desired. Lower-power alternatives to CP have previously been described in the literature (Jeener et al., 1965; Jeener and Broekaert, 1967; Redfield, 1969; Kunitomo et al., 1974; Demco et al., 1975; Emid et al., 1980; Vieth and Yannoni, 1993; Zhang et al., 1993; Kurur and Bodenhausen, 1995; Lee and Khitrin, 2008; Khitrin et al., 2011; Vinther et al., 2019), which rely on indirect polarization transfer via proton dipolar order rather than through a direct 1H–13C Hartman–Hahn matching condition (Hartmann and Hahn, 1962).
The population of a Zeeman eigenstate for a spin-1∕2 nucleus at thermal equilibrium is given as follows:
where ωi is the energy of the state for the spin of interest, T is the temperature and Z is a canonical partition function. In the high-temperature limit, the spin density operator (which describes the state of an entire ensemble of spin-1∕2 nuclei at thermal equilibrium) is expressed by using a truncated Taylor series:
where , ω0 is the nuclear Larmor frequency for the spins of interest and is the z-angular momentum operator for spin i. The second term in Eq. (2) corresponds to longitudinal magnetization. However, outside of the high-temperature approximation higher-order terms in the spin density operator expansion cannot be ignored:
The third term in Eq. (3) reveals the presence of nuclear dipolar order (Fukushima and Roeder, 1981), which can in principle be prepared by generating strongly polarized spin systems, such as those established by conducting dDNP experiments (Sugishita et al., 2019). Such dipolar order can also be efficiently generated by suitable rf-pulse sequences and ultimately used to transfer polarization (Jeener et al., 1965; Jeener and Broekaert, 1967; Redfield, 1969; Kunitomo et al., 1974; Demco et al., 1975; Emid et al., 1980; Vieth and Yannoni, 1993; Zhang et al., 1993; Kurur and Bodenhausen, 1995; Lee and Khitrin, 2008; Khitrin et al., 2011; Vinther et al., 2019). For the sake of simplicity, we will refer here to such polarization transfer schemes as dCP for dipolar order mediated cross-polarization.
In this article, we revisit the concept of 1H→13C dCP polarization transfer and assess its efficiency in the context of dDNP experiments at 1.2 K and 7.05 T. We show that for a sample of [1-13C]sodium acetate, a 13C polarization of ∼8.7 % can be achieved after ∼10 min of 1H DNP and the use of a sole polarization transfer step. The overall dCP transfer efficiency is ∼0.43 with respect to the most sophisticated and efficient high-power CP sequences available today. The experimental data presented indicate that 1H Zeeman order () is first converted to 1H–1H dipolar order () and presumably subsequently converted to the desired 13C Zeeman order (). We show how the use of microwave gating (Bornet et al., 2016b) is key to dCP as it improves the overall efficiency by a factor of more than ∼2.3.
2.1 Sample preparation and freezing
A solution of 3 M [1-13C]sodium acetate in the glass-forming mixture -d8 ( ) was doped with 50 mM TEMPOL radical (all compounds purchased from Sigma Aldrich) and sonicated for ∼10 min. This sample is referred to as I from here onwards. Paramagnetic TEMPOL radicals were chosen to most efficiently polarize 1H spins under dDNP conditions. A 100 µL volume of I was pipetted into a Kel-F sample cup and inserted into a 7.05 T prototype Bruker Biospin polarizer equipped with a specialized dDNP probe and running TopSpin 3.7 software. The sample temperature was reduced to 1.2 K by submerging the sample in liquid helium and reducing the pressure of the variable temperature insert (VTI) towards ∼0.7 mbar.
2.2 Dynamic nuclear polarization
The sample was polarized by applying microwave irradiation at 197.648 GHz (positive lobe of the EPR line) with triangular frequency modulation of amplitude Δfµw=120 MHz (Bornet et al., 2014a) and rate fmod=0.5 kHz at a power of ca. 100 mW, which were optimized prior to commencing experiments to achieve the best possible level of 1H polarization. Microwave gating was employed shortly before and during dDNP transfer experiments to allow the electron spin ensemble to return to a highly polarized state, which happens on the timescale of the longitudinal electron relaxation time (typically T1e=100 ms with Pe=99.93 % under dDNP conditions) (Bornet et al., 2016b). Consequently, the 1H and 13C relaxation times in the presence of a rf field are extended by orders of magnitude, allowing spin-locking rf pulses to be much longer, which significantly increases the efficiency of nuclear polarization transfer.
2.3 Pulse sequences
In 1967 Jeener and Broekaert established the original rf-pulse sequence for creating and observing dipolar order in the solid state (Jeener and Broekaert, 1967). Since then, other rf-pulse sequences have been proposed in the literature, usually with improved efficiency (Jeener et al., 1965; Redfield, 1969; Kunitomo et al., 1974; Demco et al., 1975; Emid et al., 1980; Vieth and Yannoni, 1993; Zhang et al., 1993; Kurur and Bodenhausen, 1995; Lee and Khitrin, 2008; Khitrin et al., 2011; Vinther et al., 2019). Herein, we are most interested in the rf-pulse sequence introduced by Vieth and Yannoni (Vieth and Yannoni, 1993) which is particularly simple, easily generates proton dipolar order and allows subsequent conversion to 13C polarization. Figure 1 shows this sequence adapted for our dDNP experiments. An electron-nuclear variant of this rf-pulse sequence has also been developed (Macho et al., 1991; Buntkowsky et al., 1991).
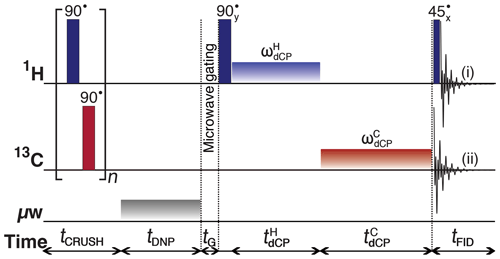
Figure 1Schematic representation of the dCP rf-pulse sequence used for preparing and monitoring 1H–1H dipolar order in I, and the conversion to 13C transverse magnetization. The experiments used the following parameters, chosen to maximize magnetization-dipolar order interconversion: n=250; tDNP=5 s; tG=0.5 s; kHz; µs; kHz; ms. The 1H and 13C continuous wave rf pulses have phase x. The π∕2 crusher rf pulses use a 13-step phase cycle to remove residual magnetization at the beginning of each experiment: , 5π∕18, π∕2, 4π∕9, 5π/18, 8π∕9, π, 10π∕9, 13π∕9, π∕18, 5π∕3, 35π∕18}. The resonance offset was placed at the centre of the 1H and 13C NMR peaks.
The dCP rf-pulse sequence operates as follows:
- i.
A crusher sequence of 90∘ rf pulses with alternating phases separated by a short delay (typically 11 ms) repeated n times (typically n=250) kills residual magnetization on both rf channels.
- ii.
The microwave source becomes active for a time tDNP during which 1H DNP builds up.
- iii.
The microwave source is deactivated and a delay of duration tG=0.5 s occurs before the next step, thus permitting the electron spins to relax to their highly polarized thermal equilibrium state (Bornet et al., 2016b).
- iv.
A 1H 90∘ rf pulse followed by a π∕2 phase-shifted continuous wave 1H rf pulse of amplitude and length converts 1H Zeeman polarization into 1H–1H dipolar order.
- v.
A 13C square rf pulse of amplitude and length presumably converts the 1H–1H dipolar order into 13C transverse magnetization.
The NMR signal can be detected by using either of the following: (i) a 1H 45∘ rf pulse followed by 1H FID acquisition to monitor the remaining proton dipolar order; or (ii) 13C FID detection to observe the converted magnetization; see Fig. 1.
The dCP rf-pulse sequence can be used in several variants.
Variant no. 1: efficiency of 1H–1H dipolar order preparation.
- a.
1H observation by fixing ms and varying and (Fig. 2a);
- b.
13C observation by fixing and (typically to an optimal value) and varying and (Fig. 2c).
Variant no. 2: efficiency of 1H–1H dipolar order conversion to 13C magnetization.
- a.
13C observation by fixing and (typically to an optimal value) and varying and (Fig. 3a);
- b.
1H observation by fixing and (typically to an optimal value) and varying and (Fig. 4a).
The amplitudes of the 1H and 13C dCP rf pulses ( and , respectively) were optimized iteratively until the intensity of the resulting NMR signals could not be improved further; see the Supplement for more details.
In the case of proton rf-channel acquisition, data points were acquired with a two-step phase cycle in which the phase of the 90y rf pulse and the digitizer were simultaneously changed by 180∘ in successive transients to remove spurious signals generated by longitudinal magnetization accrued during the dCP rf pulses. A dispersive lineshape was observed as a result of the phase cycle, which is characteristic of dipolar spin order. The resulting 1H NMR spectrum was phase corrected to yield an absorptive lineshape.
3.1 1H–1H dipolar order preparation
1H monitored optimization for the generation of 1H–1H dipolar order as a function of the dCP 1H rf-pulse duration was performed by using variant no. 1a of the dCP sequence shown in Fig. 2a. Experimental results demonstrating the preparation of 1H–1H dipolar order under variant no. 1a of the dCP sequence are shown in Fig. 2b. The integrals plotted were acquired directly on the 1H rf channel using kHz either with or without microwave gating (black circles and grey squares, respectively). In both cases, the NMR signal grows until a maximum signal intensity, which corresponds to the optimal preparation of proton dipolar order, is reached at µs, after which the signal decays towards a stable plateau on a longer timescale. However, in the case where microwave gating is removed, the signal intensity is reduced. This is due to depolarization (microwave saturation) of the electron spins, resulting in a detrimental enhancement of the paramagnetic relaxation contribution to nuclear spin relaxation. These results suggest that microwave gating improves the conversion of 1H magnetization to 1H–1H dipolar order by a factor of at least ∼1.6.
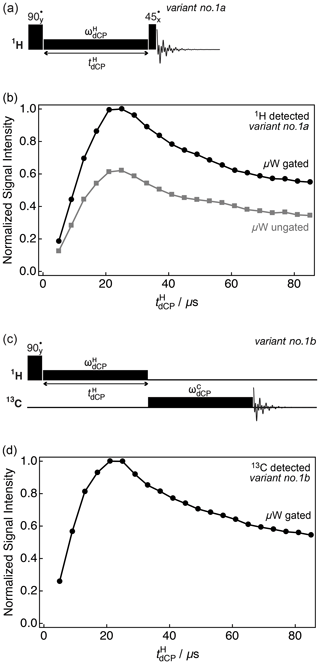
Figure 2Simplified schematic representations of (a) variant no. 1a and (c) variant no. 1b of the dCP rf-pulse sequence. Experimental (b) 1H and (d) 13C NMR signal intensities of I as a function of the 1H dCP rf-pulse duration acquired at 7.05 T (1H nuclear Larmor frequency =300.13 MHz, 13C nuclear Larmor frequency =75.47 MHz) and 1.2 K. The experiments in (b) were acquired with two transients per data point, whilst the experiments in (d) were acquired with a single transient per data point. The traces have the same overall form and plateau over a period of 200 µs (data not shown).
13C-monitored optimization for the build-up of 1H–1H dipolar order was performed by using variant no. 1b of the dCP rf-pulse sequence demonstrated in Fig. 2c. In Fig. 2d the experimental integrals are plotted against the dCP 1H rf-pulse duration and were acquired on the 13C rf channel with kHz, kHz and ms (black circles). It is important to note that the maximum is identical whether the NMR signal is observed on the 1H rf channel by using variant no. 1a or on the 13C rf channel by using variant no. 1b, and that more generally the two traces have the same shape and optimum. This shows that 13C transverse magnetization from dCP is proportional to the 1H–1H dipolar order initially prepared.
3.2 1H–13C polarization transfer
Figure 3b shows how 13C magnetization is built up by employing variant no. 2a of the dCP rf-pulse sequence; see Fig. 3a. The experimental integrals of the 13C signal are plotted against the 13C dCP rf-pulse duration with (black circles) and without (grey squares) microwave gating.
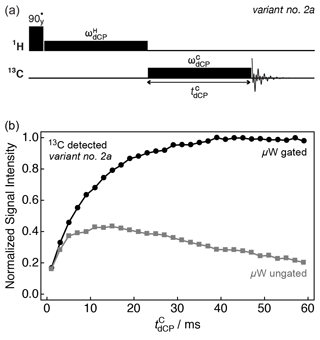
Figure 3(a) Simplified schematic representation of variant no. 2a of the dCP rf-pulse sequence. (b) Experimental 13C NMR signal intensity of I as a function of the dCP rf-pulse duration acquired at 7.05 T (1H nuclear Larmor frequency =300.13 MHz; 13C nuclear Larmor frequency =75.47 MHz) and 1.2 K with a single transient per data point.
The black trace corresponds to the growth of the 13C NMR signal. A maximum is reached at ms, with kHz. The polarization transfer efficiency is relatively robust with respect to the amplitude of the 13C dCP rf-pulse ; see the Supplement for more details. A wildly different behaviour is observed in the case where the microwave source is not gated. In this instance, a maximum signal intensity occurs at ms, with the detectable 13C signal decreasing past this point. The ratio between the maximum data points is ∼2.3, and indicates a large 13C enhancement afforded by microwave gating.
It is worth noting that the duration of the 13C dCP rf-pulse is considerably longer, more than 3 orders of magnitude, than the 1H dCP rf-pulse length. Reasons for this are examined in the discussion section below.
Figure 4b details how in variant no. 2b of the dCP rf-pulse sequence (Fig. 4a) the 1H NMR signal vanishes as the 13C dCP rf-pulse generates 13C transverse magnetization. The experimental integrals of the 1H-detected NMR signals are plotted against the 13C dCP rf-pulse duration with (black open circles) and kHz (black circles) both with microwave gating, and with kHz (grey squares) without microwave gating.
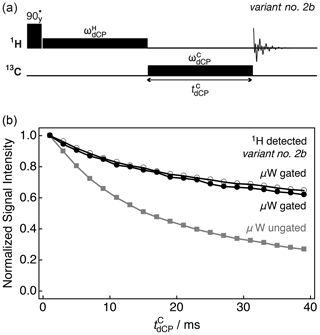
Figure 4(a) Simplified schematic representation of variant no. 2b of the dCP rf-pulse sequence. (b) Experimental 1H NMR signal intensity of I as a function of the 13C dCP rf-pulse duration acquired at 7.05 T (1H nuclear Larmor frequency =300.13 MHz; 13C nuclear Larmor frequency =75.47 MHz) and 1.2 K with two transients per data point. The experimental traces were recorded by using the following amplitudes for the 13C dCP rf-pulse : black open circles: kHz; black filled circles: kHz; grey squares: kHz. All signal amplitudes were normalized to the first data point.
The curves show how 1H–1H dipolar order decays towards thermal equilibrium mainly through relaxation and is not significantly affected by the presence of the 13C dCP rf-pulse generating 13C magnetization. The difference between the two black traces might however indicate the quantity of 1H–1H dipolar order converted into 13C magnetization. The small difference is just a few percent, indicating that only a very small portion of the 1H–1H dipolar order might be used (and be useful) to produce hyperpolarized 13C magnetization. This could be explained by the large excess of 1H spins compared with 13C spins in our sample (a factor of ∼6.2). A lack of microwave gating (grey squares) significantly compromises the generation of 13C polarization, as seen in Fig. 3b.
3.3 Comparison to cross-polarization
The performance efficiency of the dCP rf-pulse sequence was compared to a traditional CP experiment (Hartmann and Hahn, 1962; Pines et al., 1972; Perez Linde, 2009; Jannin et al., 2011; Bornet et al., 2012, 2013; Batel et al., 2012; Vuichoud et al., 2016; Cavaillès et al., 2018), which is described in the Supplement along with a rf-pulse sequence diagram and all optimized parameters. Experiments employed 640 s of direct 1H DNP at 1.2 K prior to polarization transfer to the 13C heteronucleus.
The power requirements for polarization transfer are dependent upon the rf-pulse sequence used and the capabilities of the dDNP probe. In general, the peak power for the 13C dCP rf-pulse is ∼5.4 times lower than required for CP. However, the 13C dCP rf-pulse is active for a duration ∼5.6 times longer than that of CP, and hence the overall deposited rf-pulse energy is approximately the same for both rf-pulse sequences. Notwithstanding, the moderately lower 13C dCP rf-pulse power is highly advantageous, e.g. a decreased likelihood of probe arcing events within the superfluid helium bath. The benefit of employing the dCP rf-pulse sequence becomes even more apparent when examining the proton rf-pulse durations needed for 1H–13C polarization transfer. Although the peak powers of both rf-pulse sequences are similar, the duration of the 1H dCP rf-pulse is a factor of 280 times shorter than that recommended for adequate CP. This is advantageous in the case that the B1 field produced by the dDNP probe is weak (e.g. due to large sample constraints) or is unstable at higher 1H rf-pulse powers for sufficiently long durations.
The CP rf-pulse sequence achieved a 13C polarization level of P(13C) ≃20.4 % after a single CP contact. 13C polarization levels in excess of 60 % are anticipated by using a multiple CP contact approach (Perez Linde, 2009; Jannin et al., 2011; Bornet et al., 2012, 2013; Batel et al., 2012; Vuichoud et al., 2016; Cavaillès et al., 2018). In comparison, the integral of the dCP-filtered NMR signal maximum is scaled by a factor of ∼0.43, indicating a 13C polarization of P(13C) ≃8.7 %. This is consistent with previous results reported in the literature (Perez Linde, 2009; Vinther et al., 2019). Strategies to further improve the dCP efficiency are presented in the discussion section.
The results presented in Fig. 2b and d show how the achieved 13C polarization is directly proportional to the quantity of 1H–1H dipolar order initially prepared by the 1H dCP rf-pulse. However, even if the 13C polarization closely follows the shape of the proton dipolar order creation profile, this does not constitute irrefutable proof that the 13C polarization originates from the proton dipolar order reservoir itself. Other, more complex forms of nuclear spin order might be involved. Moreover, it is feasible that an intermediate reservoir exists, such as non-Zeeman spin order of the 13C heteronucleus.
As seen in Fig. 3b, it is interesting to note that the duration of the 13C dCP rf-pulse is considerably longer, more than 3 orders of magnitude, than the 1H dCP rf-pulse duration. The reason is the relative sizes of the dipolar couplings which control the preparation and transfer processes of 1H–1H dipolar order. The generation of dipolar order involves only proton spins, which possess a magnetogyric ratio ∼4 times greater than for 13C spins and consequently larger dipolar couplings, which scale as the product of the magnetogyric ratios for the two spins involved. This results in a short time to convert 1H magnetization to 1H–1H dipolar order. Conversely, the supposed transfer of 1H–1H dipolar order to 13C nuclei would certainly demand 1H–13C dipolar couplings.
The duration of the 13C dCP rf-pulse is a factor of ∼5.6 longer than required for optimized conventional CP (see the Supplement for more details). The extended duration of the 13C dCP rf-pulse could be conceivably explained by assuming that the 1H spins closest to the 13C spin do not participate in the polarization transfer process since the 1H–1H dipolar order preparation is perturbed by the presence of the 13C spin during the 1H dCP rf-pulse. It is also possible that two dipolar coupled protons are separated by a difference in chemical shift which matches the frequency of a 13C spin in the rotating frame allowing an exchange of energy. Such events are similar to the cross-effect in DNP (Kessenikh et al., 1963), but are likely to be of lower probability, leading to an increased 13C dCP rf-pulse duration.
Not only is the polarization transfer process long, but it is also weaker than what is usually realized with optimized CP, since we obtain P(13C) ≃8.7 % rather than P(13C) ≃20.4 % in a single CP step on the same sample. Although the amplitude and duration of the proton dipolar order creation rf-pulse were carefully optimized before experimental implementation, we nevertheless believe there is still room for improvement in preparing high quantities of proton dipolar order. The performance of the dCP rf-pulse sequence could be enhanced by adopting the following strategies: (i) employing shaped rf-pulses; (ii) implementing a multiple dCP transfer approach; (iii) optimizing the protonation level of the DNP solution; (iv) exploiting deuterated molecular derivatives; (v) avoiding large quantities of methyl groups which may act as dipolar order relaxation sinks due to their inherent rotation (which remains present at liquid helium temperature); and (vi) changing the molecule [1-13C]sodium acetate for another spin system with different 1H–13C coupling strengths (e.g. simply using [2-13C]sodium acetate).
Today's performances on our current “standard” DNP sample are rather poor compared to CP; however, there are reasons to think that further improvements through advanced rf-pulse schemes and revised sample formulations will be possible in the future, and that dCP may become a viable alternative to CP. This will be particularly relevant to the cases of the following: (i) issues related to probe arcing in the superfluid helium bath which precludes the use of conventional CP experiments; (ii) increased sample volumes, e.g. in human applications; and (iii) hyperpolarization of insensitive nuclear spins: e.g. 89Y nuclei cannot be polarized easily via traditional CP experiments due to unfeasible CP matching conditions on the heteronuclear rf channel. Other alternatives to the CP approach also exist but are theoretically less efficient, such as low magnetic field nuclear thermal-mixing (Gadian et al., 2012) which relies on energy conserving mutual spin flips in overlapping NMR lineshapes to polarize heteronuclei in solid samples (Peat et al., 2016).
Dipolar order mediated 1H→13C polarization transfer occurs by employing rf-pulse methods which operate under dDNP conditions. This supposedly involves an intermediate reservoir of dipolar order, which governs the polarization transfer process. The spin dynamics of dCP were found to significantly depend on the presence of microwave gating. A maximum 13C polarization of ∼8.7 % was observed after ∼10 min of microwave irradiation and a lone polarization transfer step, which corresponds to a dCP polarization transfer efficiency of ∼0.43 with respect to optimized conventional CP. These results are promising for future applications of polarization conversion methods in the context of low-power 1H→X polarization transfer to insensitive nuclei (in particular for very low magnetogyric ratios), with minimized probe arcing and potentially large sample volumes, paving the way to the use of 1H→X polarization transfer in clinical (human-dose) contexts and for nuclear spins generally less accessible by dDNP such as 15N or 89Y.
Experimental data are available upon request from the corresponding author.
The supplement related to this article is available online at: https://doi.org/10.5194/mr-1-89-2020-supplement.
SJE performed experiments and co-wrote the manuscript; SFC, QC, OC and AB performed experiments; MC built parts of the experimental apparatus; and SJ conceived the idea and co-wrote the manuscript.
The authors declare that they have no conflict of interest.
The authors are grateful to Bruker Biospin for providing the prototype dDNP polarizer, and particularly Dmitry Eshchenko, Roberto Melzi, Marc Rossire, Marco Sacher and James Kempf for scientific and technical support. The authors additionally thank Gerd Buntkowsky (Technische Universitat Darmstadt) who kindly communicated data associated with prior publications to us; Burkhard Luy (Karlsruhe Institute of Technology) for enlightening discussions; Catherine Jose and Christophe Pages for use of the ISA Prototype Service; and Stéphane Martinez of the UCBL mechanical workshop for machining parts of the experimental apparatus.
This research was supported by ENS-Lyon, the French CNRS, Lyon 1 University, the European Research Council under the European Union's Horizon 2020 research and innovation programme (ERC grant agreement no. 714519/HP4all and Marie Skłodowska-Curie grant agreement no. 766402/ZULF), and Bruker BioSpin.
This paper was edited by Patrick Giraudeau and reviewed by two anonymous referees.
Ardenkjær-Larsen, J.-H., Fridlund, B., Gram, A., Hansson, G., Hansson, L., Lerche, M. H., Servin, R., Thaning, M., and Golman, K.: Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR, P. Natl. Acad. Sci. USA, 100, 10158–10163, https://doi.org/10.1073/pnas.1733835100, 2003.
Batel, M., Krajewski, M., Däpp, A., Hunkeler, A., Meier, B. H., Kozerke, S., and Ernst, M.: Cross-Polarization for dissolution dynamics nuclear polarization, Chem. Phys. Lett., 554, 72–76, https://doi.org/10.1039/C4CP02696A, 2012.
Bornet, A., Melzi, R., Jannin, S., and Bodenhausen, G.: Cross Polarization for Dissolution Dynamic Nuclear Polarization Experiments at Readily Accessible Temperatures 1.2 < T < 4.2, Appl. Magn. Reson., 43, 107–117, https://doi.org/10.1007/s00723-012-0358-1, 2012.
Bornet, A., Melzi, R., Perez Linde, A. J., Hautle, P., van den Brandt, B., Jannin S., and Bodenhausen, G.: Boosting Dissolution Dynamic Nuclear Polarization by Cross Polarization, J. Chem. Phys. Lett., 4, 111–114, https://doi.org/10.1021/jz301781t, 2013.
Bornet, A., Milani, J., Vuichoud, B., Perez Linde, A. J., Bodenhausen, G., and Jannin, S.: Microwave frequency modulation to enhance Dissolution Dynamic Nuclear Polarization, Chem. Phys. Lett., 602, 63–67, https://doi.org/10.1016/j.cplett.2014.04.013, 2014a.
Bornet, A., Ji, X., Mammoli, D., Vuichoud, B., Milani, J., Bodenhausen, G., and Jannin, S.: Long-Lived States of Magnetically Equivalent Spins Populated by Dissolution-DNP and Revealed by Enzymatic Reactions, Chem. Eur. J., 20, 17113–17118, https://doi.org/10.1002/chem.201404967, 2014b.
Bornet, A., Maucourt, M., Deborde, C., Jacob, D., Milani, J., Vuichoud, B., Ji, X., Dumez, J.-N., Moing, A., Bodenhausen, G., Jannin, S., and Giraudeau, P.: Highly Repeatable Dissolution Dynamic Nuclear Polarization for Heteronuclear NMR Metabolomics, Anal. Chem., 88, 6179–6183, https://doi.org/10.1021/acs.analchem.6b01094, 2016a.
Bornet, A., Pinon, A., Jhajharia, A., Baudin, M., Ji, X., Emsley, L., Bodenhausen, G., Ardenkjær-Larsen, J.-H., and Jannin, S.: Microwave-gated dynamic nuclear polarization, Phys. Chem. Chem. Phys., 18, 30530–30535, https://doi.org/10.1039/C6CP05587G, 2016b.
Buntkowsky, G., Stehlik, D., Vieth, H.-M., and Salikhov, K. M.: Nanosecond time resolution of electron-nuclear cross polarization within the optical nuclear polarization (ONP) process, J. Phys., 3, 6093–6111, https://doi.org/10.1088/0953-8984/3/32/015, 1991.
Cavaillès, M., Bornet, A., Jaurand, X., Vuichoud, B., Baudouin, D., Baudin, M., Veyre, L., Bodenhausen, G., Dumez, J.-N., Jannin, S., Copéret, C., and Thieuleux, C.: Tailored Microstructured Hyperpolarizing Matrices for Optimal Magnetic Resonance Imaging, Angew. Chem. Int. Edit., 130, 7575–7579, https://doi.org/10.1002/anie.201801009, 2018.
Demco, D. E., Tegenfeldt, J., and Waugh, J. S.: Dynamics of cross relaxation in nuclear magnetic double resonance, Phys. Rev. B, 11, 4133–4151, https://doi.org/10.1103/PhysRevB.11.4133, 1975.
Dumez, J.-N., Milani, J., Vuichoud, B., Bornet, A., Lalande-Martin, J., Tea, I., Yon, M., Maucourt, M., Deborde, C., Moing, A., Frydman, L., Bodenhausen, G., Jannin, S., and Giraudeau, P.: Hyperpolarized NMR of plant and cancer cell extracts at natural abundance, Analyst, 140, 5860–5863, https://doi.org/10.1039/C5AN01203A, 2015.
Emid, S., Konijnendijk, J., Smidt, J., and Pines, A.: On the short time behavior of the dipolar signal in relaxation measurements by the pulse method, Physica B+C, 100, 215–218, https://doi.org/10.1016/0378-4363(80)90008-X, 1980.
Fukushima, E. and Roeder, S. B. W.: Experimental Pulse NMR: A Nuts and Bolts Approach, CRC Press, Boca Raton, 1981.
Gadian, D. G., Panesar, K. S., Perez Linde, A. J., Horsewill, A. J., Köckenberger, W., and Owers-Bradley, J. R.: Preparation of highly polarized nuclear spin systems using brute-force and low-field thermal mixing, Phys. Chem. Chem. Phys., 14, 5397–5402, https://doi.org/10.1039/C2CP23536F, 2012.
Hartmann, G., Hubert, D., Mango, S., Morehouse, C. C., and Plog, K.: Proton polarization in alcohols at 50 kG, 1 K, Nucl. Instrum. Meth. A, 106, 9–12, https://doi.org/10.1016/0029-554X(73)90039-6, 1973.
Hartmann, S. R. and Hahn, E. L.: Nuclear Double Resonance in the Rotating Frame, Phys. Rev., 128, 204–2053, https://doi.org/10.1103/PhysRev.128.2042, 1962.
Jannin, S., Bornet, A., Colombo, S., and Bodenhausen, G.: Low-temperature cross polarization in view of enhancing dissolution Dynamic Nuclear Polarization in NMR, Chem. Phys. Lett., 517, 234–236, https://doi.org/10.1016/j.cplett.2011.10.042, 2011.
Jeener, J. and Broekaert, P.: Nuclear Magnetic Resonance in Solids: Thermodynamic Effects of a Pair of rf Pulses, Phys. Rev., 157, 232–240, https://doi.org/10.1103/PhysRev.157.232, 1967.
Jeener, J., Du Bois, R., and Broekaert, P.: ”Zeeman” and ”Dipolar” Spin Temperatures during a Strong rf Irradiation, Phys. Rev., 139, A1959–A1961, https://doi.org/10.1103/PhysRev.139.A1959, 1965.
Ji, X., Bornet, A., Vuichoud, B., Milani, J., Gajan, D., Rossini, A. J., Emsley, L., Bodenhausen, G., and Jannin, S.: Transportable Hyperpolarized Metabolites, Nat. Commun., 8, 13975, https://doi.org/10.1038/ncomms13975, 2017.
Kessenikh, A. V., Lushchikov, V. I., Manenkov, A. A., and Taran, Y. V.: Proton polarization in irradiated polyethylenes, Sov. Phys.-Sol. State, 5, 321–329, 1963.
Khitrin, A. K., Xu, J., and Ramamoorthy, A.: Cross-correlations between low-γ nuclei in solids via a common dipolar bath, J. Magn. Reson., 212, 95–101, https://doi.org/10.1016/j.jmr.2011.06.015, 2011.
Kunitomo, M., Hatanaka, H., and Hashi, T.: Adiabatic demagnetization in the rotating frame by non-resonant oscillating field, Phys. Lett. A, 49, 135–136, https://doi.org/10.1016/0375-9601(74)90705-1, 1974.
Kurur, N. D. and Bodenhausen, G.: Adiabatic Coherence Transfer in Magnetic Resonance of Homonuclear Scalar-Coupled Systems, J. Magn. Reson. Ser. A, 114, 163–173, https://doi.org/10.1006/jmra.1995.1123, 1995.
Lee, J.-S. and Khitrin, A. K.: Thermodynamics of adiabatic cross polarization, J. Chem. Phys., 128, 114504, https://doi.org/10.1063/1.2839436, 2008.
Lipsø, K. W., Bowen, S., Rybalko, O., and Ardenkjær-Larsen, J.-H.: Large dose hyperpolarized water with dissolution-DNP at high magnetic field, J. Magn. Reson., 274, 65–72, https://doi.org/10.1016/j.jmr.2016.11.008, 2017.
Macho, V., Stehlik, D., and Vieth, H.-M.: Spin coherence effects in the electron-nuclear polarization transfer process, Chem. Phys. Lett., 180, 398–402, https://doi.org/10.1016/0009-2614(91)85139-N, 1991.
Nelson, S. J., Kurhanewicz, J., Vigneron, D. B., Larson, P. E. Z., Harzstark, A. L., Ferrone, M., van Criekinge, M., Chang, J. W., Bok, R., Park, I., Reed, G., Carvajal, L., Small, E. J., Munster, P., Weinberg, V. K., Ardenkjær-Larsen, J.-H., Chen, A. P., Hurd, R. E., Odegardstuen, L.-I., Robb, F. J., Tropp, J., and Murray, J. A.: Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate, Sci. Transl. Med., 5, 198ra108, https://doi.org/10.1126/scitranslmed.3006070, 2013.
Peat, D. T., Hirsch, M. L., Gadian, D. G., Horsewill, A. J., Owers-Bradley, J. R., and Kempf, J. G.: Low-field thermal mixing in [1-13C] pyruvic acid for brute-force hyperpolarization, Phys. Chem. Chem. Phys., 18, 19173–19182, https://doi.org/10.1039/C6CP02853E, 2016.
Perez Linde, A. J.: Application of Cross-Polarization Techniques to Dynamic Nuclear Polarisation Dissolution Experiments, PhD Thesis, University of Nottingham, UK, 2009.
Pines, A., Gibby, M., and Waugh, J.: Proton-enhanced nuclear induction spectroscopy 13C chemical shielding anisotropy in some organic solids, Chem. Phys. Lett., 15, 373–376, https://doi.org/10.1016/0009-2614(72)80191-X, 1972.
Redfield, A. G.: Nuclear spin thermodynamics in the rotating frame, Science, 164, 1015–1023, https://doi.org/10.1126/science.164.3883.1015, 1969.
Sugishita, T., Matsuki, Y., and Fujiwara, T.: Absolute 1H polarization measurement with a spin-correlated component of magnetization by hyperpolarized MAS-DNP solid-state NMR, J. Magn. Reson., 99, 20–26, https://doi.org/10.1016/j.ssnmr.2019.02.001, 2019.
Vieth, H.-M. and Yannoni, C. S.: Cross polarization in solid state NMR spectroscopy. Efficient polarization transfer via the non-Zeeman spin reservoir, Chem. Phys. Lett., 205, 153–156, https://doi.org/10.1016/0009-2614(93)89220-C, 1993.
Vinther, J. M. O., Zhurbenko, V., Albannay, M. M., and Ardenkjær-Larsen, J.-H.: Design of a local quasi-distributed tuning and matching circuit for dissolution DNP cross polarization, Solid State Nucl. Mag., 102, 12–20, https://doi.org/10.1016/j.ssnmr.2019.04.006, 2019.
Vuichoud, B., Bornet, A., de Nanteuil, F., Milani, J., Canet, E., Ji, X., Miéville, P., Weber, E., Kurzbach, D., Flamm, A., Konrat, R., Gossert, A. D., Jannin, S., and Bodenhausen, G.: Filterable Agents for Hyperpolarization of Water, Metabolites, and Proteins, Chem. Eur. J., 22, 14696–14700, https://doi.org/10.1002/chem.201602506, 2016.
Zhang, S., Stejskal, E., Fornes, R., and Wu, X.: Mismatching Cross Polarization in the Rotating Frame, J. Magn. Reson. Ser. A, 104, 177–179, https://doi.org/10.1006/jmra.1993.1206, 1993.




