the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Inter-residue through-space scalar 19F–19F couplings between CH2F groups in a protein
Yi Jiun Tan
Elwy H. Abdelkader
Iresha D. Herath
Ansis Maleckis
Gottfried Otting
Using cell-free protein synthesis, the protein G B1 domain (GB1) was prepared with uniform high-level substitution of leucine by (2S,4S)-5-fluoroleucine (FLeu1), (2S,4R)-5-fluoroleucine (FLeu2), or 5,5′-difluoroleucine (diFLeu). 19F nuclear magnetic resonance (NMR) spectra showed chemical shift ranges spanning more than 9 ppm. Through-space scalar 19F–19F couplings between CH2F groups arising from transient fluorine–fluorine contacts are readily manifested in [19F,19F]-TOCSY spectra. The 19F chemical shifts correlate with the three-bond 1H–19F couplings (3JHF), confirming the γ-gauche effect as the predominant determinant of the 19F chemical shifts of the CH2F groups. Different 3JHF couplings of different CH2F groups indicate that the rotation of the CH2F groups can be sufficiently restricted in different protein environments to result in the preferential population of a single rotamer. The 3JHF couplings also show that CH2F groups populate the different rotameric states differently in the 5,5′-difluoroleucine residues than in the monofluoroleucine analogues, showing that two CH2F groups in close proximity influence each other's conformation. Nonetheless, the 19F resonances of the Cδ1H2F and Cδ2H2F groups of difluoroleucine residues can be assigned stereospecifically with good confidence by comparison with the 19F chemical shifts of the enantiomerically pure fluoroleucines. 1H–19F nuclear Overhauser effects (NOEs) observed with water indicate hydration with sub-nanosecond residence times.
- Article
(6323 KB) - Full-text XML
-
Supplement
(1744 KB) - BibTeX
- EndNote
Proteins made with global substitution of a single amino acid type by a selectively fluorinated analogue greatly facilitate their analysis by 19F NMR spectroscopy (Sharaf and Gronenborn, 2015). Structural perturbations caused by the fluorine substitutions can be kept to a minimum if a single fluorine atom is installed in a methyl group as the resulting CH2F group has the freedom to respond to the increased spatial requirement of the C–F moiety by preferential population of those rotamers that are most readily accommodated by the chemical environment. Recently, we showed that the Escherichia coli peptidyl–prolyl isomerase B (PpiB), which contains five leucine residues, can be produced with high-level uniform substitution of leucine for (2S,4S)-5-fluoroleucine (FLeu1), (2S,4R)-5-fluoroleucine (FLeu2), or 5,5′-difluoroleucine (diFLeu; Fig. 1) using cell-free protein synthesis (Tan et al., 2024). As demonstrated by X-ray crystal structures, the structural perturbations caused by these amino acid substitutions were minimal (Frkic et al., 2024a). Furthermore, the 3JHF coupling constants were inversely correlated with the 19F chemical shifts in a first experimental confirmation of the γ-gauche effect predicted by Oldfield and co-workers based on quantum calculations (Feeney et al., 1996). In the structure of PpiB, the leucine residues are isolated from each other. In contrast, the three leucine residues of GB1 are arranged such that methyl groups of neighbouring leucine residues can make van der Waals contacts (Fig. 2). This situation may produce through-space scalar JFF (TSJFF) couplings. Scalar couplings through non-bonded interactions are common for atoms containing free electron pairs (Hierso, 2014), and a TSJFF coupling of 21 Hz has been reported between two fluorotryptophan residues in a protein (Kimber et al., 1978).
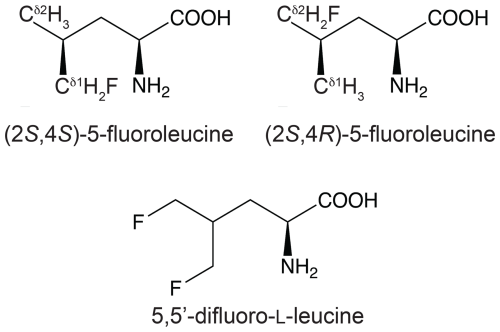
Figure 1Chemical structures of the fluorinated leucine analogues used in the present work. (2S,4S)-5-fluoroleucine (where fluorine is on the δ1 carbon), (2S,4R)-5-fluoroleucine (where fluorine is on the δ2 carbon), and 5,5′-difluoro-L-leucine are referred to in the following as FLeu1, FLeu2, and diFLeu, respectively.
1.1 Experimental procedures
1.2 Fluorinated leucine analogues
Initially, the fluorinated leucine analogues FLeu1 and FLeu2 (Fig. 1) were synthesised following published protocols (Moody et al., 1994; August et al., 1996; Charrier et al., 2004). diFLeu with and without 2H substitutions was synthesised as described (Maleckis et al., 2022). Subsequently, FLeu1, FLeu2, and diFLeu were obtained as HCl salts from Enamine (Ukraine).
1.3 Expression vectors
Expression vectors were based on pETMCSI (Neylon et al., 2000) and constructed with a C-terminal His6 tag following a TEV cleavage site. The amino terminus was preceded by the 5′-nucleotide sequence of the T7 gene 10 to ensure high expression yields, which added the hexapeptide MASMTG. The full nucleotide and amino acid sequences are shown in Table S1 in the Supplement.
1.4 Protein expression
All protein samples were expressed by continuous exchange cell-free protein synthesis (CFPS) following an established protocol (Apponyi et al., 2008; Ozawa et al., 2012). The gene of the GB1 construct was PCR-amplified with eight-nucleotide overhangs to generate circularised DNA suitable for use in CFPS (Wu et al., 2007). Leucine was omitted when preparing the acid-soluble amino acid mixture. The fluoroleucine of interest was added from an aqueous stock solution to the outer buffer at a final concentration of 4 mM. The pH of the outer buffer was adjusted to 7.5. The CFPS reaction was conducted at 30 °C for 16 h using 1 mL inner reaction mixture of S30 cell extract made from the E. coli BL21 strain and 10 mL outer buffer.
1.5 Protein purification
Proteins were purified using a 1 mL Ni–NTA gravity column (GE Healthcare, USA) equilibrated with buffer A (50 mM Tris–HCl, pH 7.5, 300 mM NaCl) using buffer B (same as buffer A but with 10 mM imidazole) for washing and buffer C (same as buffer A but with 300 mM imidazole) for elution. The purified proteins were dialysed overnight against a storage buffer (50 mM HEPES, pH 7.5, 100 mM NaCl) and concentrated using an Amicon centrifugal ultrafiltration tube with a molecular weight cut-off of 3 kDa.
1.6 Protein mass spectrometry
Intact protein mass analysis was performed using an Orbitrap Elite Hybrid Ion Trap–Orbitrap mass spectrometer equipped with an UltiMate 3000 UHPLC (Thermo Scientific, USA). The protein samples were injected via an Agilent ZORBAX SB-C3 Rapid Resolution HT Threaded Column using a 5 %–80 % gradient of acetonitrile with 0.1 % formic acid. The data were collected in positive ion mode. The protein masses were obtained by deconvolution using the Xtract function in the Qual Browser software tool of the program Xcalibur 3.0.63 (Thermo Fisher Scientific, USA).
1.7 Protein NMR conditions
All 19F NMR spectra were measured at 25 °C on a 400 MHz Bruker Avance NMR spectrometer equipped with a SmartProbe, allowing 19F detection with 1H decoupling. The protein solutions were in 90 % H2O 10 % D2O with 20 mM MES buffer, pH 6.5, and 100 mM NaCl or with 50 mM HEPES buffer, pH 7.5, and 100 mM NaCl. All spectra reported of GB1 made with diFLeu were recorded in MES buffer. All spectra reported of GB1 made with FLeu2 were recorded in HEPES buffer. All spectra reported of GB1 made with FLeu1 were recorded in HEPES buffer unless indicated otherwise. 0.1 mM trifluoroacetate (TFA) was added as an internal reference and calibrated to −75.25 ppm.
1.8 Results
1.9 Protein yields and purity
Up to 2.7 mg of protein was obtained from 1 mL inner reaction mixture of the CFPS setup (Table S2 in the Supplement). The amino acid sequence of native GB1 contains three leucine residues, and the additional TEV cleavage site present in our constructs adds a fourth leucine residue. Intact protein mass spectrometry indicated that the predominant species contained fluorinated leucine analogues at all four sites. The species containing three or two fluorinated leucine analogues were also detected, but their intensity indicated that the chance of canonical leucine at any of the four sites was below 10 % (Fig. S1 in the Supplement). Mass spectra of GB1 produced with diFLeu in the presence of some canonical leucine delivered the natural protein as the main species followed by protein containing single leucine-for-diFLeu substitutions, illustrating the strong preference of the E. coli leucyl-tRNA synthetase for L-leucine over diFLeu (Fig. S2 in the Supplement). Complete exclusion of L-leucine from the CFPS reaction could not be achieved due to amino acid impurities in the S30 extract.
1.10 Protein stability
Thermal denaturation measured by circular dichroism at 216 nm showed that the melting temperatures the GB1 samples made with fluorinated leucine analogues ranged between about 66 and 72 °C, i.e. 9–15° lower than for the wild-type protein (Fig. S3 in the Supplement), indicating that the presence of CH2F groups decreases the stability of the protein.
1.11 1D 19F NMR spectra
Figure 3 shows the 1D 19F NMR spectra of the GB1 variants produced with diFLeu (GB1-d), FLeu1 (GB1-1), or FLeu2 (GB1-2). In addition, Fig. 3b shows the spectrum of GB1 produced with diFLeu in the presence of canonical L-leucine (GB1-dd). The 1D NMR spectra resolve the signals of all fluorine atoms.
The 19F chemical shifts were insensitive to the buffer and pH but very sensitive to the immediate chemical environment. A striking illustration are the very different chemical shifts observed in GB1-d when the sample was prepared with the addition of L-leucine to produce samples predominantly containing single diFLeu residues (GB1-dd; Fig. 3a and b). Comparison of the high-field and low-field ends of the spectra of GB1-dd and GB1-d shows that minor peaks observed for the GB1-d sample correspond to main peaks observed with GB1-dd and vice versa. The minor peaks in Fig. 3a can thus be attributed to a small amount of canonical leucine in the protein preparation. Conversely, the minor peaks in the spectrum of GB1-dd appear to correspond to peaks of the fully fluorinated GB1-d sample, although the only minor species present in significant amounts contains no more than two diFLeu residues. This indicates that the presence of a second diFLeu residue is sensed only if it is in the immediate neighbourhood. Position 7 features two neighbouring leucine sites (Fig. 2), yet the Cδ1H2F group seems to sense predominantly a single neighbour, while the chemical shift of the Cδ2H2F group is less well conserved between the major species in GB1-dd and the minor species in GB1-d (Fig. 3a and b).
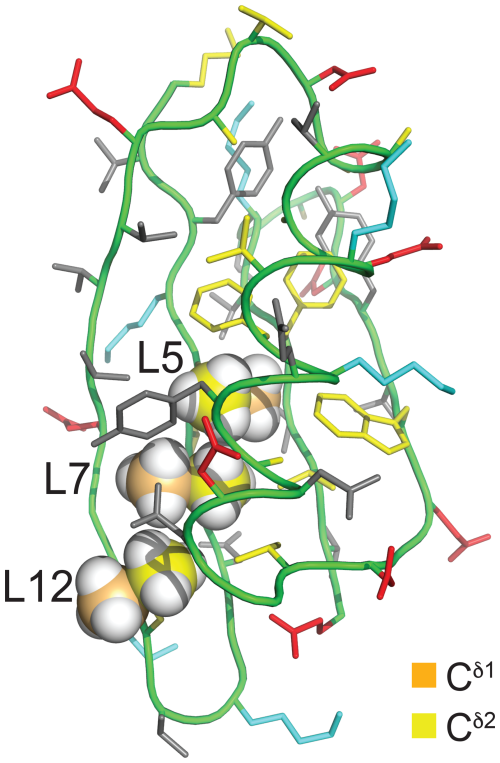
Figure 2Solution structure of GB1 (PDB ID 3GB1; Kuszewski et al., 1999). The methyl groups of Leu5, Leu7, and Leu12 are shown in a space-filling representation with the δ1- and δ2-carbon atoms in orange and yellow, respectively. The side chain of Leu5 is inaccessible to the solvent, whereas the Cδ1H3 groups of Leu7 and Leu12 are partially and highly accessible, respectively. The colour code of the other amino acids is red for negatively charged, blue for positively charged, grey for hydrophilic, and yellow for hydrophobic amino acids.
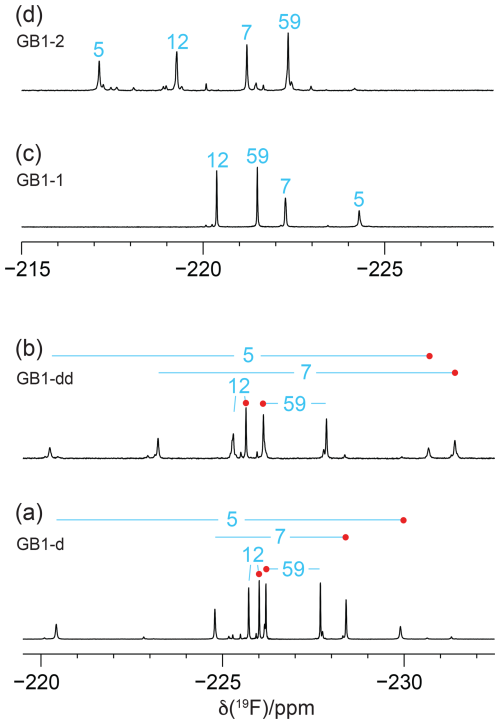
Figure 31D 19F NMR spectra of GB1 made with fluorinated leucine analogues using FLeu1 to produce GB1-1, FLeu2, for GB1-2 and diFLeu for GB1-d and GB1-dd. All spectra were recorded with 1H decoupling during acquisition using a 0.5 s recovery delay between scans. The resonance assignments are indicated by the sequence numbers of the four leucine sites. (a) GB1-d prepared with 4 mM diFLeu. Spectrum recorded of a 2 mM protein solution in 20 mM MES buffer, pH 6.5. Red dots mark the resonances assigned to Cδ1H2F groups. (b) GB1-dd prepared with a mixture of 0.5 mM leucine and 4 mM diFLeu. Spectrum recorded of a 4 mM protein solution in 20 mM MES buffer, pH 6.5. Stereospecific assignments are indicated as in (a). (c) Spectrum of a 2 mM solution of GB1-1 in 50 mM HEPES, pH 7.5. (d) Spectrum of a 2.2 mM solution of GB1-2 in 50 mM HEPES, pH 7.5.
In the case of the GB1-2 sample, minor peaks arose because the FLeu2 amino acid synthesised in house contained about 10 % FLeu1 as an impurity.
In the case of residue 59, which is in the flexible TEV protease recognition site of the C-terminal peptide segment of the protein construct, the chemical shifts are hardly impacted by the rest of the protein, as indicated by their conservation between the spectra of Fig. 3a and b. For the diFLeu residue in position 59 (Fig. 3a and b), we base the stereospecific assignment on the 19F chemical shifts observed for this position in GB1-1 and GB1-2 (Fig. 3c and d). The stereospecific assignments of the other diFLeu residues were determined by 2D NMR experiments described below. They attributed the high-field signals of residues 5, 7, and 12 to the Cδ1H2F groups. Notably, the respective signals in GB1-1 are also at higher field than the corresponding signals in GB1-2 (Fig. 3c and d).
The T1 relaxation times were of the order of 0.3 s, and the full line widths at half height ranged between 7 and 18 Hz. R1ρ(19F) relaxation rates of GB1-d indicate that no 19F signal possesses an intrinsic line width much greater than 12 Hz (Table S3 in the Supplement). For a sample produced with deuterated diFLeu, where all five protons of the isopropyl group are replaced by deuterium (Maleckis et al., 2022), the R1ρ(19F) measurements indicated a maximal intrinsic line width of 7 Hz, suggesting that dipolar relaxation by the nearest protons contributes significantly to the 19F relaxation. The broadest lines were observed for residue 5, the side chain of which is deeply buried in the core of the protein (Fig. 2), hence expected to feature the least flexibility and the fastest transverse relaxation rates. Residue 7 is the next-most-buried residue, while the side chain of residue 12 is more highly accessible to the solvent (particularly the Cδ1H2F group; see Fig. 2), and residue 59 is completely solvent-exposed.
Recording the 19F NMR spectra without decoupling of the 1H spins revealed broad multiplets with overlap between some of the resonances (Fig. 4). The multiplet of each CH2F group is composed of a triplet of doublets due to two-bond couplings, 2JHF, within each CH2F group (47 Hz) and the 3JHF coupling with the methine proton of the isopropyl group. 3JHF couplings obey a Karplus relationship (Williamson et al., 1968; Gopinathan and Narasimhan, 1971). If the 3JHF coupling is small, the envelope of the multiplet appears like a triplet, but 3JHF can also be as large as 44 Hz (Tan et al., 2024), in which case the multiplet appears like a quartet. The 19F resonances of residue 5 in GB1-1 and GB1-2 are examples of these two limiting cases (Fig. 4c and d).
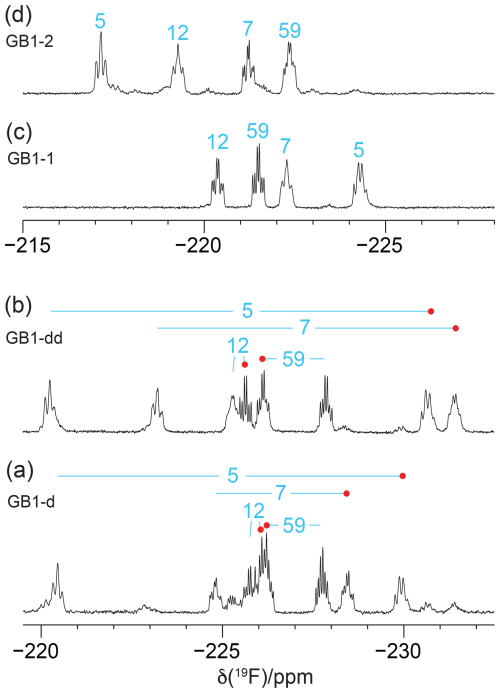
Figure 41D 19F NMR spectra without 1H decoupling recorded of the samples from Fig. 3. (a) GB1-d made with diFLeu, (b) GB1-dd made with diFLeu diluted with canonical L-leucine, (c) GB1-1 made with FLeu1, and (d) GB1-2 made with FLeu2. The resonance assignments are indicated in blue. Red dots identify stereospecific assignments of the Cδ1H2F groups in diFLeu residues.
Interestingly, the multiplet of the Cδ1H2F group of residue 12 in GB1-dd displays narrower lines than the Cδ2H2F group (Fig. 4b) in agreement with a narrower signal in GB1-1 than in GB1-2 (Fig. 3). This observation aligns with the greater solvent exposure of the Cδ1H2F group (Fig. 2). The inverse correlation between 19F NMR line width and solvent exposure suggests that faster rotation of the CH2F groups about the Cγ–Cδ bond results in slower transverse relaxation.
1.12 NMR resonance assignments
The large JHF couplings observed indicate that resonance assignments can be achieved by coherence transfer between 1H and 19F spins and linking the 1H resonances of the isopropyl groups to the backbone protons by [1H,1H]-TOCSY and [1H,1H]-NOESY spectra. The 1H chemical shifts of the CH2F groups are near 4 ppm, and the methine resonances are between 1 and 2 ppm. For GB1 made with diFLeu, a [1H,19F]-COSY spectrum connected the 19F NMR signals belonging to the same residue (Fig. 5).
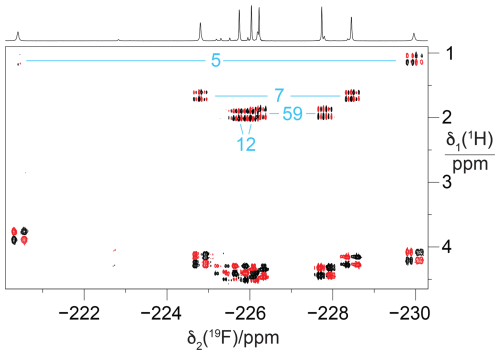
Figure 5[1H,19F] correlation spectrum of a concentrated solution of GB1-d (about 10 mM). The 1H-decoupled 1D 19F NMR spectrum is shown at the top. The [1H,19F]-COSY spectrum was recorded with the pulse sequence 90°(1H) – t1 – 90°(1H),90°(19F) – acquisition(19F). The cross peaks with the methine proton of the isopropyl groups, which identify the pairs of CH2F groups belonging to the same residue, are assigned in blue. Parameters: t1max = 51 ms, t2max = 217 ms, 1.4 h total recording time.
To probe for the presence of scalar through-space 19F–19F couplings in GB1-d, we recorded a [19F,19F]-TOCSY spectrum. The spectrum yielded both intra-residual and through-space correlations (Fig. 6a). Interestingly, the intra-residual cross peak of residue 5 could not be observed, whereas the inter-residual connectivities with the nearest neighbour (residue 7) were intense. Residue 7 in turn showed cross peaks to residues 5 and 12, which were more intense than the intra-residual cross peaks. The absence of the intra-residual cross peak of residue 5 indicates that scalar 4JFF couplings cannot be relied upon to connect the 19F NMR signals of the CH2F groups of each diFLeu residue.
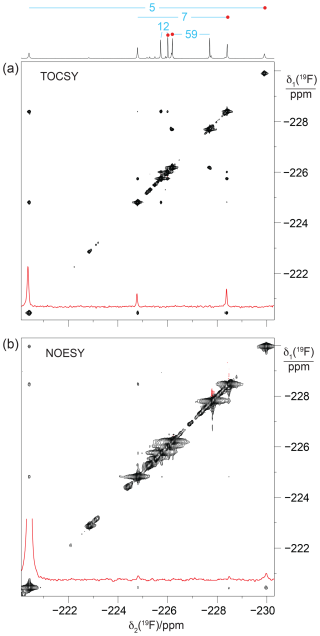
Figure 6[19F,19F]-TOCSY and [19F,19F]-NOESY spectra of GB1-d. The 1D 19F NMR spectrum is shown at the top along with the resonance assignments. Cross sections through the diagonal peaks at −220.4 ppm are shown in red. (a) [19F,19F]-TOCSY spectrum (mixing time 60 ms) recorded of an 0.8 mM protein solution. Parameters: DIPSI-2 mixing with 4200 Hz RF field strength, t1max = 26 ms, t2max = 105 ms, 14 h total recording time. (b) [19F,19F]-NOESY spectrum (mixing time 200 ms) recorded of a > 10 mM solution of GB1-d. Parameters: t1max = 13.5 ms, t2max = 108 ms, 12 h total recording time, processed with 20 Hz exponential line broadening in the δ2 dimension. Without cropping, the diagonal peak in the cross section would exceed the boundary of panel (b) twofold.
Notably, some of the most intense [19F,19F]-TOCSY cross peaks came about by TSJFF couplings. To exclude the possibility of TOCSY cross peaks arising from 19F–19F nuclear Overhauser effects (NOEs), we also recorded a [19F,19F]-NOESY spectrum (Fig. 6b). The NOESY spectrum produced the intra-residual cross peak of residue 5 with greater intensity than the inter-residual NOEs. This illustrates the different dependence of NOEs and TSJFF couplings on the internuclear distance, with TSJFF couplings depending on close contacts between the fluorine atoms to create the necessary orbital overlap. Notably, although the NOESY spectrum had been recorded of a GB1-d sample with over 10-fold-higher protein concentration, the cross-peak intensities were markedly poorer in the NOESY than in the TOCSY spectrum.
The GB1 samples prepared with FLeu1 or FLeu2 (GB1-1 and GB1-2, respectively) offer fewer opportunities for TSJFF couplings. The conformation shown in Fig. 2 excludes direct contacts between Cδ1H3 groups, whereas van der Waals contacts between 19F atoms of Cδ2H2F groups are arguably possible in view of the greater C–F bond length and larger van der Waals radius of fluorine compared with hydrogen. Even so, direct fluorine–fluorine contacts in GB1-2 depend on specific rotamer combinations of neighbouring CH2F groups and may be infrequent if the CH2F groups rotate.
Experimentally, residue 7 produced inter-residual cross peaks in the [19F,19F]-TOCSY spectra of GB1-1 and GB1-2 (Fig. 7). In the case of GB1-1, the cross peaks were about 100 times smaller than the diagonal peaks. In the case of GB1-2, residue 7 produced cross peaks both with residue 5 and residue 12, and those cross peaks were only about 10 times smaller than the diagonal peaks. No single conformation of the Cδ2H2F group of residue 7 can simultaneously engage in fluorine–fluorine contacts with residues 5 and 12 (Fig. 2), suggesting that the Cδ2H2F group populates multiple rotamers. Furthermore, the cross peak observed between the Cδ1H2F groups of residues 7 and 12 in GB1-1 suggests that these side chains enjoy greater conformational freedom than captured by the NMR structure 3GB1, which was determined with the aim of presenting the single best approximation to the average structure.
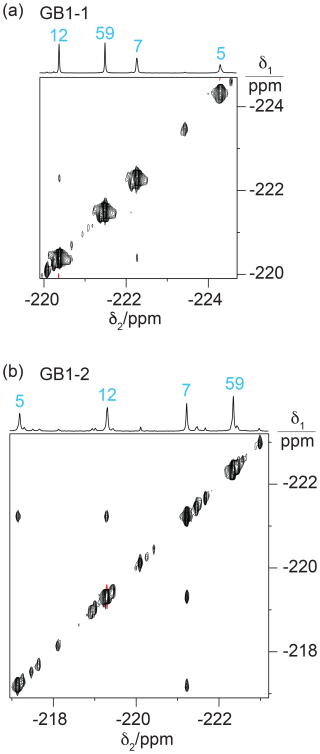
Figure 7[19F,19F]-TOCSY spectra of 2 mM solutions of GB1-1 and GB1-2 recorded with 60 ms mixing time. The 1D NMR spectra are plotted on top with the resonance assignments in blue. (a) TOCSY spectrum of GB1-1 recorded in about 3 h using t1max = 8.5 ms and t2max = 128 ms. The cross peaks between residues 7 and 12 are about 100 times weaker than the diagonal peaks. (b) TOCSY spectrum of GB1-2 recorded in about 12 h using t1max = 8 ms and t2max = 171 ms. The cross peaks are about 10 times smaller than the diagonal peaks.
The fluorine–fluorine contacts observed in GB1-2 recapitulate the two strongest cross peaks observed with the high-field 19F resonance of residue 7 in GB1-d (Fig. 6a). Assuming that the side-chain conformations are conserved between GB1-2 and GB1-d, this affords stereospecific assignments of GB1-d, assigning the high-field signals of residues 7 and 12 and the low-field signal of residue 5 to the 19F spins of the respective Cδ2H2F groups. Given this assignment, the weaker interaction between the low-field signals of residues 7 and 12 indicates a contact between a Cδ1H2F and a Cδ2H2F group, which cannot occur in either GB1-1 or GB1-2. The generally greater cross-peak intensities observed in GB1-d may be a consequence of the greater steric crowding associated with the spatial demands of multiple fluorine atoms, bringing the 19F spins into closer contact. In addition, the greater density of 19F spins in GB1-d opens the chance for multiple magnetisation transfer steps during the TOCSY mixing period.
1.13 Estimates of TSJFF from [19F,19F]-TOCSY spectra
Using the sample of GB1-d produced with deuterated diFLeu (Fig. S4a in the Supplement) to minimise the relaxation of 19F, we recorded [19F,19F]-TOCSY spectra with increasing mixing time τm (27.6, 41.4, and 55.3 ms), measured the integrals of cross peaks (IC) and diagonal peaks (ID) and calculated the ratio . In the approximation of a two-spin system and assuming that the cross peaks and diagonal peaks relax at the same rate, the ratio is expected to evolve during isotropic mixing with tan2(πJFFτm) (Braunschweiler and Ernst, 1983). The largest JFF couplings found in this way were about 2–3 Hz (Fig. S5 in the Supplement).
1.14 Heteronuclear NMR for residue assignment
Heteronuclear [1H,19F]-NOESY (HOESY) spectra recorded with 150 ms mixing times showed NOEs with nearby protons (Fig. 8). These NOEs delivered residue-specific resonance assignments as many of the corresponding 1H nuclei were also detected in conventional homonuclear [1H,1H]-NOESY spectra. For example, residue 5 in GB1-1 and GB1-d displays NOEs to a 1H resonance at about −0.8 ppm. This resonance matches a β-proton of Leu5, which in wild-type GB1 is the most high-field 1H resonance due to aromatic ring currents from Phe28. In all three samples, the 19F NMR signal of residue 5 produced stronger HOESY cross peaks than the other fluorinated leucine residues, while residue 59 delivered relatively weak cross peaks if any. This result indicates that a CH2F group produces stronger HOESY cross peaks when it is buried in the core of the protein than when it is solvent exposed and can rotate in an unhindered manner. The 19F NMR assignments of residue 7 were confirmed similarly by comparison of the cross peaks observed in the HOESY and [1H,1H]-NOESY spectra.
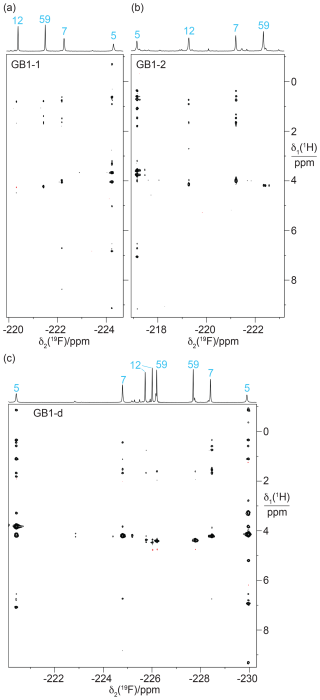
Figure 8[1H,19F]-HOESY spectra of GB1 produced with FLeu1, FLeu2, or diFLeu. The spectra were recorded with a mixing time of 150 ms. The corresponding 1D 19F NMR spectra are shown at the top along with the resonance assignments. (a) HOESY spectrum of a 2.2 mM solution of GB1-1 in MES buffer, recorded using t1max = 38 ms, t2max = 136 ms, 9.6 h total recording time. (b) HOESY spectrum of a 2 mM solution of GB1-2, recorded using t1max = 30 ms, t2max = 136 ms, 34 h total recording time. (c) HOESY spectrum of a 10 mM solution of GB1-d, recorded using t1max = 31 ms, t2max = 108 ms, 2.3 h total recording time.
In the case of the diFLeu residue in position 12, stronger cross peaks were detected for the low-field signal assigned to the Cδ2H2F than the Cδ1H2F group (Fig. 8c). In addition, the Cδ1H2F group of this residue displays a negative cross peak with the water resonance (at 4.75 ppm), as do both 19F NMR signals of residue 59, indicating intermolecular NOEs with hydration water molecules featuring sub-nanosecond residence times (Otting et al., 1991). This confirms the solvent exposure of these fluorine atoms and agrees with the stereospecific assignments of residue 12 made by comparing the TSJFF couplings with the protein structure. We observed no other negative NOE cross peaks with the CH2F groups.
Starting from the assignment of residue 5, the cross peaks observed in the [19F,19F]-TOCSY spectra provided an additional, straightforward assignment pathway for the 19F spins in GB1-d and GB1-2 (Fig. 6b and 7b). In GB1-dd, as in GB1-1, the Cδ1H2F group of residue 12 produced only weak HOESY cross peaks. The HOESY spectrum thus did not identify the 19F NMR signals belonging to the same diFLeu residue in position 12. This link, however, was easily established by correlations with the γ proton of the isopropyl group observed in a short-delay 1H,19F correlation experiment.
1.15 Measurement of 3JHF couplings and γ-gauche effect
3JHF couplings are governed by a Karplus relationship describing their dihedral angle dependence, and thus provide information about the rotameric states of the CH2F groups. Quantitative measurements of J couplings in the 1D 19F NMR spectra recorded without 1H decoupling were hampered by spectral overlap and the presence of sample heterogeneities (Fig. 3). Narrower 19F multiplets were obtained for samples prepared with diFLeu versions that had been synthesised with CD2F instead of CH2F groups (Maleckis et al., 2022), where 3JHF couplings were manifested in a sample made with diFLeu containing a CγH group, whereas these splittings were absent from a sample prepared with deuterated diFLeu containing a CγD group. A simple comparison of these spectra shows a correlation between the 19F chemical shifts and splittings due to 3JHF couplings (Fig. S4).
For more quantitative measurements of the 3JHF couplings, we recorded short-delay 1H,19F correlation experiments (Tan et al., 2024), which encode the 3JHF coupling constants in the relative peak intensities of Hγ–19F versus Hδ–19F cross peaks. The results confirm the correlation between the 3JHF coupling constants and the 19F chemical shifts (Fig. 9). This correlation is a hallmark of the γ-gauche effect, which associates a high-field 19F chemical shift with the rotameric state of the CH2F group that positions the 19F spin trans relative to the γ proton of the isopropyl group (Feeney et al., 1996; Tan et al., 2024; Frkic et al., 2024a, b). Conversely, the 19F NMR resonance is shifted low-field if the 19F spin is positioned trans relative to a carbon atom. The γ-gauche effect is most clearly illustrated by the low-field and high-field signals of residue 5.
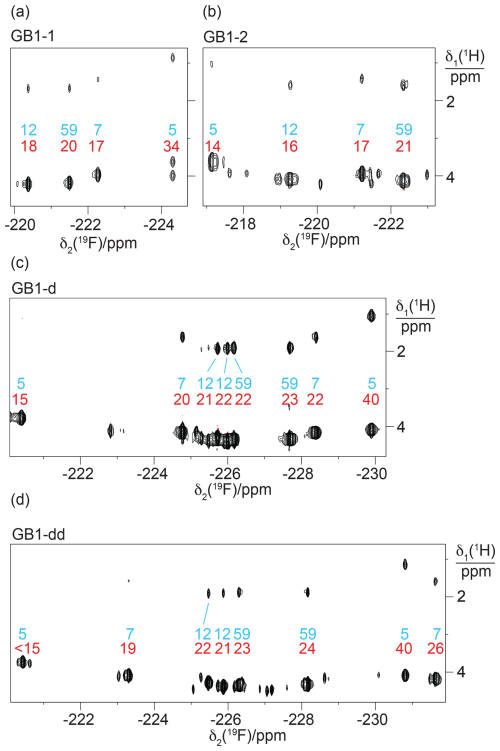
Figure 9Short-delay 1H,19F correlation experiments for the measurement of 3JHF coupling constants. The experiments were conducted with a 1H constant-time evolution period of 7 ms to evolve the JHF couplings and a refocusing INEPT period of 2.5 ms (Tan et al., 2024). The resonance assignments are indicated in blue. Red numbers indicate the 3JHF coupling constants (in Hz) derived from the relative intensities of the Hγ–19F versus Hδ–19F cross peaks. (a) Spectrum recorded of a 2 mM solution of GB1-1. Parameters: t1max = 7 ms, t2max = 128 ms, 1.3 h total recording time. (b) Spectrum recorded of a 2 mM solution of GB1-2 using t1max = 6.9 ms, t2max = 171 ms, 2.8 h total recording time. (c) Spectrum recorded of a 0.8 mM solution of GB1-d using t1max = 7 ms, t2max = 180 ms, 5.3 h total recording time. (d) Spectrum recorded of a 0.6 mM solution of GB1-dd produced with diFLeu diluted with Leu using t1max = 7 ms, t2max = 181 ms, 22.3 h total recording time.
The immediate chemical environment of the 19F spins also affects their chemical shifts. For example, the 3JHF coupling of the high-field 19F resonance of residue 7 in GB1-dd is smaller than for residue 5 (26 Hz versus 40 Hz; Table S4 in the Supplement), yet the resonance appears more high-field in the spectrum (Fig. 9d). Quite generally, the 19F chemical shifts of residue 7 are very sensitive to the presence or absence of fluorinated residues in positions 5 and 12 (Fig. 9c and d), highlighting the impact of the chemical environment.
Interestingly, the 19F spins of residue 7 showed significantly different 3JHF couplings between the GB1-d and GB1-dd preparations, suggesting somewhat different populations of the different rotameric states of the CH2F groups. The associated changes in 19F chemical shifts are high-field and low-field as predicted by the γ-gauche effect.
Very large and very small 3JHF couplings indicate that CH2F groups are trapped in pure trans or gauche rotamers, respectively, showing that the rotation of a CH2F group about the Cγ–Cδ bond axis can be halted by the steric restraints in the tightly packed core of the protein. In the case of the E. coli peptidyl–prolyl cis–trans isomerase B (PpiB) produced with FLeu and diFLeu, we determined 3JHF couplings ranging between 9 and 44 Hz (Tan et al., 2024). The 3JHF couplings observed in GB1 are less extreme, suggesting that each CH2F group populates more than a single rotamer. Using residue 59 located in the flexible TEV cleavage motif as a reference, a 3JHF coupling of about 22 Hz is indicative of a CH2F group that populates all three possible staggered rotamers. The different 3JHF couplings observed for residue 5 (Table S4), which is the most deeply buried leucine side chain in the wild-type protein, thus indicate clear conformational preferences for its CH2F groups. The largest 3JHF couplings were observed for diFLeu rather than FLeu1 or FLeu2 residues, as expected for fluorine–fluorine interactions biasing the conformational space of the CH2F groups (Marstokk and Møllendal, 1997; Wu et al., 1998; Lu et al., 2019).
On a technical note, the short-delay 1H,19F correlation experiments delivered the Hγ chemical shifts with much greater sensitivity than the [1H,19F]-COSY experiment recorded without heteronuclear decoupling (Fig. 5), assisting with the resonance assignments by comparison with [1H,1H]-NOESY spectra. In terms of sensitivity, the short-delay 1H,19F correlation experiments were also far superior to the HOESY spectra. The chemical shifts of the Hγ spins were well conserved between the samples made with FLeu1, FLeu2, and diFLeu, ascertaining the 19F resonance assignment of GB1-dd by comparison with GB1-d.
1.16 13C-NMR spectroscopy
The 13C chemical shifts of the CH3 groups in the singly fluorinated leucine analogues FLeu1 and FLeu2 were shifted upfield by between 5.6 and 8.2 ppm relative to the shifts of the methyl groups in the wild-type protein (Fig. S6 in the Supplement). Highly conserved 1H and 13C chemical shifts of the GB1 variants indicate that the 3D fold of the protein remains unchanged by the fluorinated leucine analogues. Therefore, any differences in chemical shifts reflect local rather than global effects. The 13C heteronuclear single-quantum coherence (13C-HSQC) spectra showed the cross peaks of the CH2F groups in the 13C dimension near 90 ppm for GB1-1 and GB1-2 and about 86 ppm for GB1-d (Fig. S7). In the 1H dimension, most CH2F groups displayed two different chemical shifts for the diastereotopic 1H spins, which, except for residue 5 in GB1-1, were unresolved in the short-delay 1H,19F correlation experiments (Fig. 9). The intensities of the 13C-HSQC cross peaks of the CH2F groups of residue 5 were rather weak (similar to those of CH2 groups of other buried amino acid residues), which correlates with the relatively broad 19F NMR signals observed for this residue. The other CH2F groups showed more intense 13C-HSQC cross peaks on par with solvent-exposed CH2 groups. The methyl cross peaks of Leu5 are relatively weak also in wild-type GB1 (Fig. S6; Goehlert et al., 2004).
The conformational impact of the fluorination of leucine methyl groups has previously been investigated in solution for only a single protein, PpiB, which contains five isolated leucine residues (Tan et al., 2024; Frkic et al., 2024a). The current findings recapitulate many of the findings made for PpiB.
- i.
In the case of the most buried residue, residue 5, the rotation of the CH2F groups is sufficiently hindered to bias the populations of the different rotamers in favour of a trans configuration of the Cδ1H2F group and a gauche configuration of the Cδ2H2F group as defined by the 3JHF coupling constants. The size of the 3JHF couplings indicates that these conformational biases are more pronounced in GB1-d than in GB1-1 and GB1-2, which may be attributed to unfavourable electrostatic interactions between parallel and antiparallel C–F bonds in a 1,3-difluoropropane moiety intrinsically limiting the conformational freedom (Marstokk and Møllendal, 1997; Wu et al., 1998; Lu et al., 2019).
- ii.
The large chemical shift dispersion of the 19F NMR signals over many parts per million is mainly due to the γ-gauche effect, which attributes high-field and low-field shifts to trans and gauche rotamers (Feeney et al., 1996). Intermediate chemical shifts correlate with intermediate 3JHF coupling constants and are thus indicative of averaging between different rotamers. A γ-gauche effect in leucine side chains has previously been reported also for the 13C chemical shifts of leucine CδH3 groups, which correlate with 3JCC couplings with the α-carbon (MacKenzie et al., 1996). To the best of our knowledge, the present work is only the third experimental example of the γ-gauche effect in CH2F groups (Fig. S8; Tan et al., 2024; Frkic et al., 2024b).
- iii.
The more solvent accessible CH2F groups displayed less extreme 3JHF couplings and less extreme 19F chemical shifts, suggesting more extensive averaging between different rotameric states. Larger and smaller 3JHF couplings as well as greater 19F chemical shift dispersions have been observed previously in PpiB (Tan et al., 2024), suggesting that the CH2F groups populate more than a single rotamer even in the buried residue 5.
- iv.
The line widths of the 19F NMR signals vary greatly between different residues and, most strikingly for residue 12, between different CH2F groups. Narrow signals correlate with high solvent exposure. For wild-type GB1, order parameter values determined by relaxation measurements have been reported for the methyl groups of Leu12 (< 0.15), Leu7 Cδ2H3 (0.15), and Leu5 Cδ1H3 (0.55), showing that the methyl group symmetry axes are subject to motions, which are more prominent in situations of high solvent exposure (Goehlert et al., 2004). The relatively high order parameter of Leu5 correlates with 19F NMR signals that are broader than any others.
- v.
For any given position in the protein, the relative chemical shifts observed between FLeu1 and FLeu2 are strongly predictive of the stereospecific assignments of a diFLeu residue at the same site. The same feature also prevails in PpiB (Tan et al., 2024).
The present work shows, for the first time, that through-space 19F–19F couplings can readily be detected between singly fluorinated CH2F groups in a protein. In previous work, we detected TSJFF couplings between genetically encoded CF3-phenylalanine and CF3-tyrosine residues in the core of PpiB (Orton et al., 2021). Notably, however, the 19F NMR spectra of PpiB constructs with multiple CF3 groups showed additional resonances, suggesting structural perturbations arising from the additional space requirements of CF3 groups. The 19F NMR spectra of GB1 made with fluorinated leucine analogues also display weak additional resonances, but there is no evidence that they are due to structural heterogeneity. Instead, the additional signals are consistent with chemical heterogeneity arising from incomplete substitution of canonical leucine by fluorinated leucines or incomplete optical purity of the synthesised fluoroleucine. Cell-free protein synthesis enables the requisite high level of global substitution of canonical amino acids by fluorinated analogues.
The observation of TSJFF couplings in GB1 is non-trivial as they depend on direct contact between the fluorine atoms. Crystal structures of PpiB showed that CH2F groups often populate multiple staggered rotamers that differ by rotation about the bond with the carbon atom they are bound to (Frkic et al., 2024a, b). Based on the 3D structure of wild-type GB1 (Fig. 2), only specific rotamer combinations generate fluorine–fluorine contacts. A crystal structure of ubiquitin synthesised chemically with two FLeu1 residues indicated that the lowest energy conformation avoids fluorine–fluorine contacts (Alexeev et al., 2003). In the case of 1,3-difluoropropane, it is known that the polarity of C–F bonds discourages rotamers that produce fluorine–fluorine contacts (Marstokk and Møllendal, 1997; Wu et al., 1998; Lu et al., 2019). Therefore, the privileged attraction between fluorine atoms in perfluorinated polymers such as Teflon does not govern the interaction between the single fluorine atoms of CH2F groups. Nonetheless, the transient 19F–19F contacts arising from random rotations of the CH2F groups in GB1-1, GB1-2, and GB1-d suffice to generate observable TSJFF couplings. As noted previously (Orton et al., 2021; Tan et al., 2024), the much steeper distance dependence of TSJFF couplings compared with 19F–19F NOEs (Ernst and Ibrom, 1995; Mallory et al., 2000) strongly favours the detection of transient fluorine–fluorine contacts by [19F,19F]-TOCSY rather than [19F,19F]-NOESY experiments (Fig. 6).
For the side chain of Leu12 in GB1, a very different χ2 angle has been reported by the crystal structures (1PGA, 1PGB; Gallagher et al., 1994; 2QMT; Frericks Schmidt et al., 2007) versus the NMR solution structure (3GB1; Kuszewski et al., 1999). As a result, the crystal structures expose the δ2 methyl group to the solvent, while the solution structure exposes the δ1 methyl group. The observation of 1H–19F NOEs with water together with different 19F NMR line widths indicative of more facile rotation of the Cδ1H2F than Cδ2H2F group fully agree with the conformation of Leu12 depicted in Fig. 2, indicating that fluorinated leucine residues do not alter the solution structure. Simple rotations of the CH2F groups allow for accommodating the fluorine atoms in the energetically most favourable rotamers.
Establishing sequence-specific resonance assignments of the 19F NMR spectra by 2D NMR techniques rather than site-directed mutagenesis is straightforward for small proteins like GB1. For larger proteins, site-specific selective installation of the fluorinated amino acids by genetic encoding (Orton et al., 2021; Qianzhu et al., 2020, 2022, 2024) will be helpful. Work towards this goal is in progress.
The NMR data are available at https://doi.org/10.5281/zenodo.15266133 (Otting, 2025).
The supplement related to this article is available online at https://doi.org/10.5194/mr-6-131-2025-supplement.
YJT, EHA, and IDH prepared the protein samples and performed 1D NMR measurements. AM synthesised fluorinated leucine analogues with and without deuteration. GO coordinated the project, performed the 2D NMR measurements, and prepared the final paper and figures.
At least one of the (co-)authors is a member of the editorial board of Magnetic Resonance. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We thank Eliza Tarcoveanu for initial syntheses of FLeu1 and FLeu2.
This research has been supported by the Australian Research Council (grant nos. CE200100012 and DP230100079).
This paper was edited by Michael Summers and reviewed by Ad Bax and one anonymous referee.
Alexeev, D., Barlow, P. N., Bury, S. M., Charrier, J.-D., Cooper, A., Hadfield, D., Jamieson, C., Kelly, S. M., Layfield, R., Mayer, R. J., McSparron, H., Price, N. C., Ramage, R., Sawyer, L., Starkmann, B. A., Uhrin, D., Wilken, J., and Young, D. W.: Synthesis, structural and biological studies of ubiquitin mutants containing (2S, 4S)-5-fluoroleucine residues strategically placed in the hydrophobic core, ChemBioChem, 4, 894–896, https://doi.org/10.1002/cbic.200300699, 2003.
Apponyi, M. A., Ozawa, K., Dixon, N. E., and Otting, G.: Cell-free protein synthesis for analysis by NMR spectroscopy, Methods Mol. Biol., 426, 257–268, https://doi.org/10.1007/978-1-60327-058-8_16, 2008.
August, R. A., Khan, J. A., Moody, C. M., and Young, D. W.: Stereospecific synthesis of (2S,4R)-[5,5,5-2H3]leucine, J. Chem. Soc.-Perk. T. 1, 1, 507–514, https://doi.org/10.1039/p19960000507, 1996.
Braunschweiler, L. and Ernst, R. R.: Coherence transfer by isotropic mixing – application to proton correlation spectroscopy, J. Magn. Reson., 53, 512–528, https://doi.org/10.1016/0022-2364(83)90226-3, 1983.
Charrier, J.-D., Hadfield, D. S., Hitchcock, P. B., and Young, D. W.: Synthesis of (2S,4S)- and (2S,4R)-5-fluoroleucine and (2S,4S)-[5,5-2H2]-5-fluoroleucine, Org. Biomol. Chem., 2, 474–482, https://doi.org/10.1039/b314933a, 2004.
Ernst, L. and Ibrom, K.: A new quantitative description of the distance dependence of through-space 19F, 19F spin–spin coupling, Angew. Chem. Int. Edit., 34, 1881–1882, https://doi.org/10.1002/anie.199518811, 1995.
Feeney, J., McCormick, J. E., Bauer, C. J., Birdsall, B., Moody, C. M., Starkmann, B. A., Young, D. W., Francis, P., Havlin, R. H., Arnold, W. D., and Oldfield, E.: 19F nuclear magnetic resonance chemical shifts of fluorine containing aliphatic amino acids in proteins: studies on Lactobacillus casei dihydrofolate reductase containing (2S,4S)-5-fluoroleucine, J. Am. Chem. Soc., 118, 8700–8706, https://doi.org/10.1021/ja960465i, 1996.
Frericks Schmidt, H. L., Sperling, L. J., Gao, Y. G., Wylie, B. J., Boettcher, J. M., Wilson, S. R., and Rienstra, C. M. J.: Crystal polymorphism of protein GB1 examined by solid-state NMR spectroscopy and X-ray diffraction, Phys. Chem. B, 111, 14362–14369, https://doi.org/10.1021/jp075531p, 2007.
Frkic, R. L., Tan, Y. J., Abdelkader, E. H., Maleckis, A., Tarcoveanu, E., Nitsche, C., Otting, G., and Jackson, C. J.: Conformational preferences of the non-canonical amino acids (2S,4S)-5-fluoroleucine, (2S,4R)-5-fluoroleucine, and 5,5'-difluoroleucine in a protein, Biochemistry, 63, 1388–1394, https://doi.org/10.1021/acs.biochem.4c00081, 2024a.
Frkic, R., Tan, Y. J., Maleckis, A., Otting, G., and Jackson, C. J.: 1.3 Å crystal structure of E. coli peptidyl–prolyl isomerase B with uniform substitution of valine by (2S,3S)-4-fluorovaline reveals structure conservation and multiple staggered rotamers of CH2F groups, Biochemistry, 63, 2602–2608, https://doi.org/10.1021/acs.biochem.4c00345, 2024b.
Gallagher, T, Alexander, P., Bryan, P., and Gilliland, G. L.: Two crystal structures of the B1 immunoglobulin-binding domain of streptococcal protein G and comparison with NMR, Biochemistry, 33, 4721–4729, https://doi.org/10.1021/bi00181a032, 1994.
Goehlert, V. A., Krupinska, E., Regan, L., and Stone, M. J.: Analysis of side chain mobility among protein G B1 domain mutants with widely varying stabilities, Protein Sci., 13, 3322–3330, https://doi.org/10.1110/ps.04926604, 2004.
Gopinathan, M. S. and Narasimhan, P. T.: Finite perturbation molecular orbital approach to the dihedral angle dependence of vicinal H-H and H-F couplings, Mol. Phys., 21, 1141–1144, https://doi.org/10.1080/00268977100102271, 1971.
Hierso, J.-C.: Indirect nonbonded nuclear spin–spin coupling: a guide for the recognition and understanding of “through-space” NMR J constants in small organic, organometallic, and coordination compounds, Chem. Rev., 114, 4838–4867, https://doi.org/10.1021/cr400330g, 2014.
Kimber, B. J., Feeney, J., Roberts, G. C. K., Birdsall, B., Griffiths, D. V., Burgen, A. S. V., and Sykes, B. D.: Proximity of two tryptophan residues in dihydrofolate reductase determined by 19F NMR, Nature, 271, 184–185, https://doi.org/10.1038/271184a0, 1978.
Kuszewski, K., Gronenborn, A. M., and Clore, G. M.: Improving the packing and accuracy of NMR structures with a pseudopotential for the radius of gyration, J. Am. Chem. Soc., 121, 2337–2338, https://doi.org/10.1021/ja9843730, 1999.
Lu, T., Zhang, J., Chen, J., Gou, Q., Xia, Z., and Feng, G.: Structure and non-covalent interactions of 1,3-difluoropropane and its complex with water explored by rotational spectroscopy and quantum chemical calculations, J. Chem. Phys., 150, 064305, https://doi.org/10.1063/1.5079564, 2019.
MacKenzie, K. R., Prestegard, J. H., and Engelman, D. M.: Leucine side-chain rotamers in a glycophorin A transmembrane peptide as revealed by three-bon carbon–carbon couplings and 13C chemical shifts, J. Biomol. NMR, 7, 256–260, https://doi.org/10.1007/BF00202043, 1996.
Mallory, F. B., Mallory, C. W., Butler, K. E., Lewis, M. B., Xia, A. Q., Luzik, E. D., Fredenburgh, L. E., Ramanjulu, M. M., Van, Q. N., Francl, M. M., Freed, D. A., Wray, C. C., Hann, C., Nerz-Stormes, M., Carroll, P. J., and Chirlian, L. E.: Nuclear spin–spin coupling via nonbonded interactions. 8. The distance dependence of through-space fluorine–fluorine coupling, J. Am. Chem. Soc., 122, 4108–4116, https://doi.org/10.1021/ja993032z, 2000.
Maleckis, A., Abdelkader, E. H., Herath, I. D., and Otting, G.: Synthesis of fluorinated leucines, valines and alanines for use in protein NMR, Org. Biomol. Chem., 20, 2424–2432, https://doi.org/10.1039/D2OB00145D, 2022.
Marstokk, K.-M. and Møllendal, H.: Structural and conformational properties of 1,3-difluoropropane as studied by microwave spectroscopy and ab initio calculations, Acta Chem. Scand., 51, 1058–1065, https://doi.org/10.3891/acta.chem.scand.51-1058, 1997.
Moody, C. M., Starkmann, B. A., and Young, D. W.: Synthesis of (2S,4S)-5-fluoroleucine, Tetrahedron Lett., 35, 5485–5488, https://doi.org/10.1016/S0040-4039(00)73531-3, 1994.
Neylon, C., Brown, S. E., Kralicek, A. V., Miles, C. S., Love, C. A., and Dixon, N. E.: Interaction of the Escherichia coli replication terminator protein (Tus) with DNA: a model derived from DNA-binding studies of mutant proteins by surface plasmon resonance, Biochemistry, 39, 11989–11999, https://doi.org/10.1021/bi001174w, 2000.
Orton, H. W., Qianzhu, H., Abdelkader, E. H., Tan, Y. J., Habel, E. I., Frkic, R. L., Jackson, C. J., Huber, T., and Otting, G.: Through-space scalar 19F–19F couplings between fluorinated non-canonical amino acids for the detection of specific contacts in proteins, J. Am. Chem. Soc., 143, 19587–19598, https://doi.org/10.1021/jacs.1c10104, 2021.
Otting, G.: NMR spectra of GB1 produced with fluorinated leucine along with spectra of the wild-type reference, Zenodo [data set], https://doi.org/10.5281/zenodo.15266133, 2025.
Otting, G., Liepinsh, E., and Wüthrich, K.: Protein hydration in aqueous solution, Science, 254, 974–980, https://doi.org/10.1126/science.1948083, 1991.
Ozawa, K., Loscha, K. V., Kuppan, K. V., Loh, C. T., Dixon, N. E., and Otting, G.: High yield cell-free protein synthesis for site-specific incorporation of unnatural amino acids at two sites, Biochem. Biophys. Res. Commun., 418, 652–656, https://doi.org/10.1016/j.bbrc.2012.01.069, 2012.
Qianzhu, H., Welegedara, A. P., Williamson, H., McGrath A. E., Mahawaththa, M. C., Dixon, N. E., Otting, G., and Huber, T.: Genetic encoding of para-pentafluorsulfanyl phenylalanine: a highly hydrophobic and strongly electronegative group for stable protein interactions, J. Am. Chem. Soc., 142, 17277–17281, https://doi.org/10.1021/jacs.0c07976, 2020.
Qianzhu, H., Abdelkader, E. H., Herath, I. D., Otting, G., and Huber, T.: Site-specific incorporation of 7-fluoro-L-tryptophan into proteins by genetic encoding to monitor ligand binding by 19F NMR spectroscopy, ACS Sens., 7, 44–49, https://doi.org/10.1021/acssensors.1c02467, 2022.
Qianzhu, H., Abdelkader, E. H., Otting, G., and Huber, T.: Genetic encoding of fluoro-L-tryptophans for site-specific detection of conformational heterogeneity in proteins by NMR spectroscopy, J. Am. Chem. Soc., 146, 13641–13650, https://doi.org/10.1021/jacs.4c03743, 2024.
Sharaf, N. G., and Gronenborn, A. M.: 19F-modified proteins and 19F-containing ligands as tools in solution NMR studies of protein interactions, Methods Enzymol., 565, 67–95, https://doi.org/10.1016/bs.mie.2015.05.014, 2015.
Tan, Y. J., Abdelkader, E. H., Tarcoveanu, E., Maleckis, A., Nitsche, C., and Otting, G.: (2S,4S)-5-Fluoroleucine, (2S,4R)-5-fluoroleucine, and 5,5'-difluoroleucine in E. coli PpiB: protein production, 19F NMR, and ligand sensing enhanced by the γ-gauche effect, Biochemistry, 63, 1376–1387, https://doi.org/10.1021/acs.biochem.4c00080, 2024.
Williamson, K. L., Hus, Y.-F. L., Hall, F. H., Swager, S., and Coulter, M. S.: Dihedral angle and bond angle dependence of vicinal proton–fluorine spin–spin coupling, J. Am. Chem. Soc., 90, 6717–6722, https://doi.org/10.1021/ja01026a028, 1968.
Wu, D., Tian, A., and Sun, H.: Conformational properties of 1,3-difluoropropane, J. Phys. Chem. A, 102, 9901–9905, https://doi.org/10.1021/jp982164w, 1998.
Wu, P. S. C., Ozawa, K., Lim, S. P., Vasudevan, S. G., Dixon N. E., and Otting, G.: Cell-free transcription/translation from PCR-amplified DNA for high-throughput NMR studies, Angew. Chem. Int. Edit., 46, 3356–3358, https://doi.org/10.1002/anie.200605237, 2007.




