the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Solid-state 1H spin polarimetry by 13CH3 nuclear magnetic resonance
Stuart J. Elliott
Quentin Stern
Sami Jannin
Dissolution dynamic nuclear polarization is used to prepare nuclear spin polarizations approaching unity. At present, 1H polarization quantification in the solid state remains fastidious due to the requirement of measuring thermal equilibrium signals. Line shape polarimetry of solid-state nuclear magnetic resonance spectra is used to determine several useful properties regarding the spin system under investigation. In the case of highly polarized nuclear spins, such as those prepared under the conditions of dissolution dynamic nuclear polarization experiments, the absolute polarization of a particular isotopic species within the sample may be directly inferred from the characteristics of the corresponding resonance line shape. In situations where direct measurements of polarization are complicated by deleterious phenomena, indirect estimates of polarization using coupled heteronuclear spins prove informative. We present a simple analysis of the 13C spectral line shape of [2-13C]sodium acetate based on the normalized deviation of the centre of gravity of the 13C peaks, which can be used to indirectly evaluate the proton polarization of the methyl group moiety and very likely the entire sample in the case of rapid and homogeneous 1H–1H spin diffusion. For the case of positive microwave irradiation, 1H polarization was found to increase with an increasing normalized centre of gravity deviation. These results suggest that, as a dopant, [2-13C]sodium acetate could be used to indirectly gauge 1H polarizations in standard sample formulations, which is potentially advantageous for (i) samples polarized in commercial dissolution dynamic nuclear polarization devices that lack 1H radiofrequency hardware, (ii) measurements that are deleteriously influenced by radiation damping or complicated by the presence of large background signals and (iii) situations where the acquisition of a thermal equilibrium spectrum is not feasible.
- Article
(1272 KB) - Full-text XML
-
Supplement
(2487 KB) - BibTeX
- EndNote
Classical nuclear magnetic resonance (NMR) experiments produce inherently weak signals. The severely limiting low intrinsic sensitivity of the technique can be enhanced by up to 4 orders of magnitude by employing a wide range of routinely used hyperpolarization methodologies (Ardenkjær-Larsen et al., 2003; Hirsch et al., 2015; Dale and Wedge, 2016; Meier, 2018; Kouřil et al., 2019). The significantly boosted NMR signal intensities from metabolites hyperpolarized by implementing a dissolution dynamic nuclear polarization (dDNP) approach have been used in the characterization of cancer in human patients (Nelson et al., 2013; Chen et al., 2020; Gallagher et al., 2020).
To hyperpolarize nuclear spins via the dDNP approach, the spin system of interest is co-frozen in a mixture of aqueous solvents and glassing agents with a carefully chosen paramagnetic radical species (Abragam and Goldman, 1978). The dDNP-compatible solution is subsequently frozen at liquid-helium temperatures (where the solvent matrix forms a glass) inside a magnetic field and is irradiated with slightly off-resonant (with respect to the centre of electron spin transition) microwaves, which transfer the high electron spin polarization to the nuclear spins of interest (Kundu et al., 2019).
Hyperpolarization of methyl group moieties by dDNP has led to some unusual effects including the generation of long-lived spin order, which is revealed in the liquid state upon dissolution of the material from cryogenic conditions (Meier et al., 2013; Roy et al., 2015; Dumez et al., 2017; Elliott et al., 2018). Solid-state NMR of highly polarized nuclear spins has previously been utilized to infer the sample polarization level and, in suitable cases, the quantity of long-lived spin order established (Waugh et al., 1987; Kuhns et al., 1989; Marohn et al., 1995; Kuzma et al., 2013; Mammoli et al., 2015; Willmering et al., 2017; Elliott et al., 2018; Aghelnejad et al., 2020). To the best of our knowledge, the solid-state NMR spectra of strongly polarized methyl groups have not shown any significant features which may be used for a clear line shape analysis.
In this communication, we propose that the 13C NMR line shape of [2-13C]sodium acetate can be used to indirectly quantify the 1H polarization of the methyl group spins. Furthermore, since 1H–1H spin diffusion rapidly achieves a homogeneous proton polarization across the entire sample, the 1H polarization level of the whole sample is therefore likely to be reflected by the 1H polarization of the methyl group moiety. We analyse the experimental 13C NMR spectra acquired for different 1H polarizations and herein present a straightforward approach to indirectly quantify the 1H polarization based on the 13C NMR peak normalized deviation of the centre of gravity (CoG). 1H polarization was observed to increase with an increasing 13C NMR peak CoG deviation (case of positive microwave irradiation).
2.1 Sample preparation
A solution of 3 M [2-13C]sodium acetate in the glass-forming mixture H2O/D2O/glycerol-d8 (1/3/6 ) was doped with 50 mM TEMPOL radical (all compounds purchased from Sigma-Aldrich) and sonicated for ∼ 10 min. Paramagnetic TEMPOL radicals were chosen to polarize 1H spins most efficiently under our dDNP conditions.
2.2 Sample freezing
A 100 µL volume of the above sample was pipetted into a Kel-F sample cup and inserted into a 7.05 T prototype Bruker Biospin polarizer equipped with a specialized dDNP probe, including a background-free radiofrequency (rf) coil insert (Elliott et al., 2021a), running TopSpin 3.5 software. The sample temperature was reduced to 1.2 K by submerging the sample in liquid helium and reducing the pressure of the variable temperature insert (VTI) towards ∼ 0.7 mbar.
2.3 Dynamic nuclear polarization
The 100 µL of sample was polarized by applying microwave irradiation at fμw=197.616 GHz (positive lobe of the DNP enhancement profile) or fμw=198.192 GHz (negative lobe of the DNP enhancement profile) with triangular frequency modulation (Bornet et al., 2014) of amplitude MHz or MHz, respectively, and rate fmod=0.5 kHz at a power of ca. 125 mW at the output of the microwave source (value given by the provider of our microwave source VDI/AMC 705) and ca. 30 mW reaching the DNP cavity (evaluated by monitoring the helium bath pressure; see Sect. 2.4), which were optimized prior to commencing experiments to achieve the highest possible level of 1H polarization.
2.4 Microwave power evaluation
The microwave power reaching the DNP cavity was determined by comparison with the heating from a resistor in the liquid helium bath and calibrating how much the bath pressure increases vs. microwave power. In practice, the measurement was performed as follows:
- i.
The VTI was filled with liquid helium and pumped down to 0.65 mbar, corresponding to 1.2 K.
- ii.
The change of pressure when turning on a resistive heater or the microwave source for 120 s was monitored. The pressure plateaus after approximatively 60 s.
- iii.
The pressure difference between the base pressure and that under the effect of the resistive heater or the microwave source ΔPmbar is calculated.
All measurements were performed ensuring that the liquid helium level in the VTI was not varying by more than a few centimetres: the microwave cavity was immersed under 5–10 cm of liquid helium. The measurements performed using the resistive heater with power Pheater are used to plot a calibration curve Pheater vs. ΔPmbar with slope a. The deposited microwave power in the cavity is then obtained by computing Pmicrowave=aΔPmbar.
2.5 Polarization build-ups
To monitor 13C NMR spectral line shapes with satisfactory signal-to-noise ratios (SNRs), 13C polarization must first be built-up by using a succession of optimized cross-polarization (CP) contact rf pulses. Then, to observe changes in the line shape of 13C NMR spectra acquired as the 1H polarization builds up from the thermal to DNP equilibrium, we employed a series of 1H saturating rf pulses followed by microwave activation, a small flip-angle rf pulse and 13C NMR signal detection, as shown by the rf pulse sequence shown in Fig. 1. The build-up of 13C polarization throughout the microwave irradiation period was tracked by engaging the following experimental procedure:
- i.
A saturating sequence of 90∘ rf pulses with alternating phases separated by a short delay (typically (typ.) 11 ms) repeated n times (typ. n=50) kills residual magnetization on both rf channels.
- ii.
The microwave source becomes active and 1H polarization builds up.
- iii.
The 13C Zeeman magnetization trajectory is minimally perturbed by the application of a small flip-angle rf pulse (typ. β = 3.5∘) used for detection, which is then followed by a short acquisition period (typ. tFID = 1 ms).
- iv.
1H DNP builds up during a time (typ. = 30 s).
- v.
Stages (iii)–(iv) are cycled m times (typ. m = 6) in order to monitor the evolution of the 13C polarization (between CP steps).
- vi.
The microwave source is gated, and a delay of duration tG = 0.5 s occurs (see Sect. 2.6), thus permitting the electron spins to relax to their highly polarized thermal equilibrium state before the next CP step (Bornet et al., 2016).
- vii.
Two synchronized adiabatic half-passages (AHPs) simultaneously produce transverse magnetization for all pulsed spin species.
- viii.
The nuclear magnetization is subsequently spin-locked on both rf channels (typically by a high-power rf pulse with a nutation frequency of the order of 15 kHz and a duration between 1–10 ms) and 1H → 13C polarization transfer occurs (Bornet et al., 2016).
- ix.
A second pair of harmonized AHPs (operating with reverse chronology) restores Zeeman magnetization on each rf channel.
- x.
Stages (ii)–(ix) are repeated in L units (typ. L = 8) to periodically transfer 1H Zeeman polarization to 13C nuclear spins.
- xi.
A second saturating sequence of 90∘ rf pulses with alternating phases separated by a short delay (typ. 11 ms) repeated n times (typ. n = 50) kills residual magnetization on the 1H rf channel only.
- xii.
The microwave source reactivates.
- xiii.
The 13C Zeeman magnetization trajectory is minimally perturbed by the application of a small flip-angle rf pulse (typ. β = 3.5∘) used for detection, which is then followed by a short acquisition period (typ. tFID = 1 ms).
- xiv.
1H DNP builds up during a time (typ. = 5 s).
- xv.
Stages (xiii)–(xiv) are cycled p times (typ. p = 80) to monitor the evolution of the 13C NMR spectra as a function of the 1H polarization build-up with sufficient SNR.
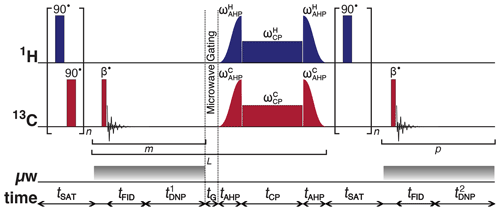
Figure 1Schematic representation of the rf pulse sequence used to accrue 13C polarizations and monitor 13C line shapes as a function of the 1H polarization. The experiments used the following key parameters chosen to maximize the efficiency of the rf pulse sequence: n = 50; β = 3.5∘; m = 6; = 30 s; L = 8; tG = 0.5 s; p = 80; and = 5 s. AHP = adiabatic half-passage. AHP sweep width = 100 kHz. The saturating rf pulses used an empirically optimized 13-step phase cycle to remove residual magnetization at the beginning of each experiment: {0, , , , , , , π, , , , , . The resonance offset was placed at the most intense peak of the 1H and 13C NMR spectra.
Further details regarding multiple-contact CP rf pulse sequence operation are given elsewhere (Bornet et al., 2016). It should be stressed that the use of CP is purely optional, and in most cases its use will be dictated by the rf hardware available. We use CP here simply as a means to offer greater SNRs for 13C NMR signal detection. Given the level of sample deuteration, at 6.7 T and with microwave modulation suitable SNRs can also be achieved with direct 13C DNP (Cheng et al., 2013).
Since it is unlikely that the 13C NMR line shape is significantly influenced by the 13C polarization, we can afford not to diminish the 13C NMR signal intensity by a sequence of 13C saturating rf pulses on the 13C rf channel at stage (xi) to maintain high SNRs. The small rf pulse flip angles are necessary to preserve the 1H and 13C polarizations throughout the course of the build-up experiment.
2.6 Microwave gating
Microwave gating was employed shortly before and during CP experiments to allow the electron spin ensemble to return to a highly polarized state, which happens on the timescale of the longitudinal electron relaxation time (typ. T1e = 100 ms with Pe = 99.93 % under our experimental dDNP conditions) (Bornet et al., 2016). Microwave gating hence provides a way to strongly attenuate paramagnetic relaxation, and consequently the 1H and 13C T1ρ relaxation time constants in the presence of an rf field are extended by orders of magnitude. This allows spin-locking rf pulses to be much longer, which significantly increases the efficiency of nuclear polarization transfer.
3.1 13C CP build-ups and decays
The CP build-up curves for the 13C polarizations PC as a function of the 1H DNP time tDNP for both positive and negative microwave irradiation are shown in Fig. 2. The 13C polarizations PC were accrued by employing the rf pulse sequence shown in Fig. 1. The 13C polarizations PC ultimately reached PC ≃ 40.6 % and PC ≃ −46.8 % after 8 CP transfers and 24 min of positive and negative microwave irradiation, respectively. The achieved levels of 13C polarization PC are lower than those previously reported in the literature (Bornet et al., 2016) but were not further optimized since only the 13C NMR line shape was of interest in this study as a probe for absolute 1H polarization. This is inconsequential for the current study since sufficient SNRs of the order of ∼ 965 and ∼ 1244 were achieved for the cases of positive and negative microwave irradiation, respectively. After this point, i.e., beyond the vertical dashed line (1H DNP time = 24 min), a slow and partial decay of the 13C NMR signal intensity towards a pseudo-equilibrium is observed; see Fig. 2. This 13C NMR signal decay is not a problem in general since the 13C NMR signal remains sufficiently intense as to allow clear measurement of the 13C NMR line shape with high accuracy.
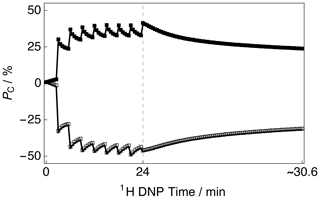
Figure 2Experimental 13C polarization PC CP build-up curves and subsequent 13C signal decays as a function of 1H DNP time acquired at 7.05 T (1H nuclear Larmor frequency = 300.13 MHz, 13C nuclear Larmor frequency = 75.47 MHz) and 1.2 K with a single transient per data point. The presented data were acquired by using the rf pulse sequence depicted in Fig. 1. Black filled squares: positive microwave irradiation; black empty squares: negative microwave irradiation. The vertical dashed line denotes the 1H DNP time at which the 1H NMR signal was destroyed by a second series of saturating rf pulses (as shown by the rf pulse sequence illustrated in Fig. 1).
3.2 13C NMR spectra
Figure 3 shows the relevant part of the experimental 13C NMR spectra acquired with a small flip angle rf pulse (β = 3.5∘) at two different 1H DNP times. The 13C NMR spectra in Fig. 3 were acquired by using the rf pulse sequence shown in Fig. 1. The initial 13C NMR spectrum (acquired at 24 min) has a linewidth at full-width half-maximum height (FWHM) of ∼ 10.9 kHz. The 13C NMR line shape is relatively symmetrical and has no obvious defining features; see Fig. 3a. Small peak contributions to the 13C NMR spectrum are observed towards the baseline, including one environment shifted as much as ca. −300 ppm. This spectrum corresponds to a low level of 1H polarization ( ≃ 0 %).
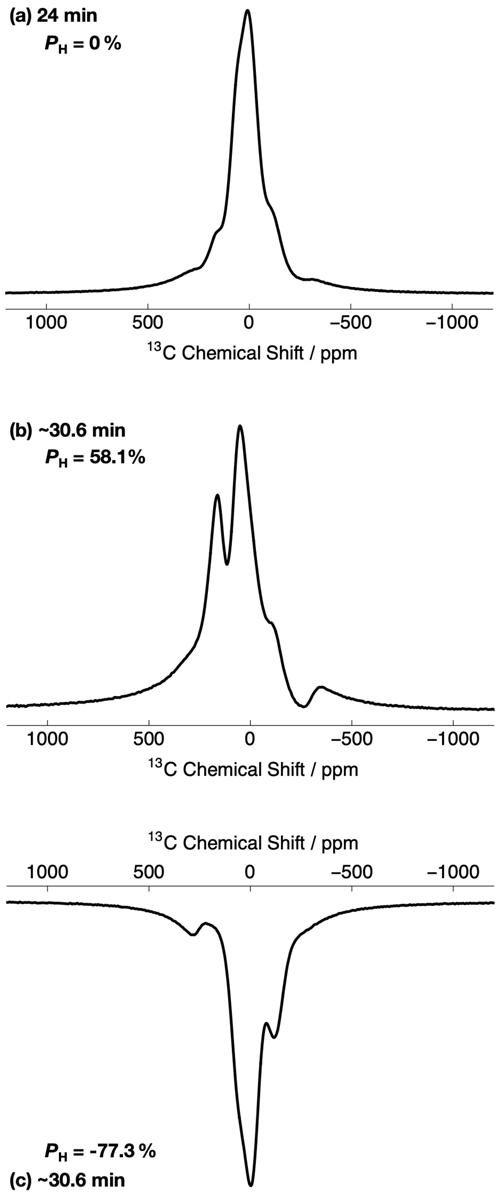
Figure 3Relevant portions of the experimental 13C NMR spectra belonging to the 13C-labelled methyl group (13CH3) of [2-13C]sodium acetate acquired at 7.05 T (1H nuclear Larmor frequency = 300.13 MHz, 13C nuclear Larmor frequency = 75.47 MHz) and 1.2 K with a single transient (rf pulse flip angle = 3.5∘) at two different 1H DNP times. The labels indicate the 1H DNP times at which the spectra were recorded. The timings coincide with those shown in Fig. 2. The 13C NMR spectra were acquired by using the rf pulse sequence depicted in Fig. 1. (a) No microwave irradiation; (b) positive microwave irradiation; and (c) negative microwave irradiation. All 13C NMR spectra have been scaled to yield the same maximum intensity.
However, the 13C NMR spectra become more complicated and gain sharper spectral features at extended 1H DNP times; see Fig. 3b and c. At ∼ 30.6 min, the 13C NMR spectra are comprised of (at least) two main resonances with differing NMR signal intensities. In the case of positive microwave irradiation (Fig. 3b), the frequency separation between the two most intense 13C NMR peaks is ∼ 8.4 kHz, and the linewidth at FWHM is ∼ 17.7 kHz. It is interesting to note that the 13C NMR spectra acquired in the cases of positive (Fig. 3b) and negative (Fig. 3c) microwave irradiation do not have the same overall profile at long 1H DNP times. These spectra correspond to much higher levels of 1H polarization ( %).
3.3 13C NMR peak normalized centre of gravity deviation vs. 1H polarization
The DNP build-up curve for the 1H polarization PH as a function of the 1H DNP time for positive microwave irradiation is shown in Fig. 4. More details regarding how to acquire such build-up curves are given in the following reference (Elliott et al., 2021b). The 1H polarization build-up curve was found to have a stretched exponential behaviour, and the experimental data are well fitted with a stretched exponential function using a 1H DNP build-up time constant denoted . Stretched exponential function: , where A is a constant, is the 1H DNP build-up time constant extracted from the above fitting procedure and β is the breadth of the distribution of 1H DNP build-up time constants. The mean 1H DNP build-up time constant is calculated as follows: , where is the gamma function. A similar 1H polarization build-up curve for the case of negative microwave irradiation, with parameters and , is shown in the Supplement.
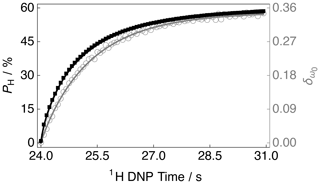
Figure 4Experimental 1H polarization PH DNP build-up curve (black filled squares and left-hand axis) and 13C NMR peak CoG normalized deviation (grey empty circles and right-hand axis) as a function of the 1H DNP time acquired at 7.05 T (1H nuclear Larmor frequency = 300.13 MHz, 13C nuclear Larmor frequency = 75.47 MHz) and 1.2 K with a single transient per data point for the case of positive microwave irradiation. The timings coincide with those shown in Fig. 2. The black solid line indicates the best fit of the experimental data points for the 1H polarization PH DNP build-up curve, and has the corresponding fitting function: . Mean 1H DNP build-up time constant s.
The sample polarized to PH ≃ −77.3 % (1H DNP time ≃ 30.6 min) by employing negative microwave irradiation with a 1H DNP build-up time constant of = 122.0 ± 0.4 s (β = 0.87). A reduced 1H polarization of PH ≃ 58.1 % was reached (at 1H DNP time ≃ 30.6 min) by using positive microwave irradiation. The 1H DNP build-up time constant was much shorter in this case: = 80.2 ± 0.3 s (β = 0.77).
The 13C NMR line shapes presented in Fig. 3 are complicated and so it is desirable to construct a parameter which can describe the 1H polarization PH, be robust with respect to field inhomogeneities and easily applied to any line shape. Figure 4 therefore also displays the 13C NMR peak CoG deviation as a function of the 1H DNP time for the case of positive microwave irradiation. The 13C NMR peak CoG normalized deviation is defined as
where Masym is denoted as the first moment of asymmetry and corresponds to the following quantity:
The first moment of asymmetry Masym is based on a calculation whereby the CoG of the 13C NMR peak ω0 is held constant at ω0(PH=0 %), i.e., the 13C NMR peak CoG corresponding to when the 1H polarization PH is zero. The CoG of the 13C NMR peak ω0 is calculated as
where the intensities of the 13C NMR peaks are normalized as follows:
where ω is the resonance frequency, and f(ω) is the peak intensity at ω. The procedure outlined above ensures that Masym=0 at PH = 0 % such that the described approach can be readily generalized to any line shape. The quantity LW0 is a measure of the linewidth of the 13C NMR peak in the case ofPH = 0 %:
i.e., the square root of the second moment at PH = 0 %. This factor establishes a 13C NMR peak CoG deviation (defined in Eq. 1) which is a normalized and dimensionless quantity.
Figure 4 indicates that at longer 1H DNP times, where the 1H polarization PH is higher, there is a greater 13C NMR peak CoG normalized deviation . Similar curves to those presented in Fig. 4 for the case of negative microwave irradiation are shown in the Supplement. It should be noted that the curve profiles and final values of are not mirror images of each other. This is also reflected in the 13C NMR spectra acquired at ∼ 30.6 min; see Fig. 3. The rate of change in the value of during the first ∼ 100 s of Fig. 4 indicates a more rapid change in the 1H polarization PH. This coincides with the starkest changes in 13C NMR line shape; see the Supplement.
The 13C NMR peak CoG normalized deviation as a function of the 1H polarization PH for positive microwave irradiation is shown in Fig. 5. The 1H polarization PH increases with an increasing 13C NMR peak CoG normalized deviation. The experimental data were fitted with a phenomenological relationship of the kind: , where is the 1H polarization as a function of the 13C NMR peak CoG normalized deviation , β is the order of the polynomial fit, and A is a scaling factor. The phenomenological function is simply used to correlate the 13C NMR peak CoG normalized deviation with the 1H polarization PH. The best fit values of the phenomenological function to the experimental data over the range of 13C NMR peak CoG normalized deviations shown in Fig. 5 are given in the caption.
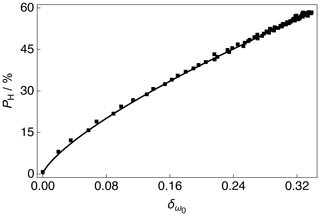
Figure 5Experimental 1H polarizations PH as a function of the 13C NMR peak CoG normalized deviation acquired at 7.05 T (1H nuclear Larmor frequency = 300.13 MHz, 13C nuclear Larmor frequency = 75.47 MHz) and 1.2 K with a single transient per data point for the case of positive microwave irradiation. The experimental data were fitted with a phenomenological function: . The best fit values are A = 129.1 % ± 0.8 % and β = 0.736 ± 0.005. The absolute 1H polarizations PH were measured by comparison with a thermal equilibrium 1H NMR signal.
As discussed in Sect. 3.3, the CoG normalized deviation of the peaks in the 13C NMR spectrum indirectly provide the level of 1H polarization PH; see Fig. 5. It is unlikely that a uniform spin temperature between the 1H and 13C nuclear spin reservoirs is reached at any time during the experiment presented in Fig. 1, but as long as a uniform spin temperature is achieved within the 1H nuclear spin reservoir then the methodology presented above holds. It should be noted that the order of the polynomial fit β shown in Fig. 5 is likely to be influenced by the capabilities of the rf probe, such as the rf pulse homogeneity, and it is therefore recommended that (if possible) users implement similar measurements on their own experimental setups rather than simply reusing the value presented here. In this way, any laboratory can adopt the procedure and reproduce the result.
Once the 13C NMR peak CoG normalized deviation falls below zero the 1H polarization PH rapidly drops towards negative values; see the Supplement. This result implies that the NMR peak CoG normalized deviation is less sensitive to negative microwave irradiation. This change in sensitivity of the 13C NMR peak CoG normalized deviations to positive and negative microwave irradiation is also evident in the 13C NMR spectra; see Fig. 3 and the Supplement. This is likely associated with the following: (i) 13C NMR spectra at negative levels of 1H polarization have line shapes with less pronounced features, i.e., partially unresolved peaks, and (ii) the 13C NMR line shape changes less dramatically as a function of negative 1H polarization. These points could both be related to NMR line narrowing due to radiation damping for the case of negative microwave irradiation (Mao and Ye, 1997; Krishnan and Murali, 2013).
1H polarizations in the range of 0 % ≲ PH ≲ 30 % typically correspond to those accrued by 1H DNP build-up experiments performed at liquid helium temperatures of 3.8–4.2 K. These results indicate that the 13C NMR peak CoG normalized deviation can therefore also be used to infer 1H polarizations PH accurately at elevated temperatures. However, the presence of methyl group rotation at temperatures above 1.2 K is likely to somewhat average the 1H–13C dipolar couplings and could lead to a different trend compared with the fit presented in Fig. 5 (Latanowicz, 2005).
One possible contribution to the inflexion in the fit of the 13C NMR peak CoG normalized deviations at low levels of 1H polarization PH is the presence of strong polarization gradients or highly polarized clusters of nuclear spins located within specific radii of the electron spins within the sample at short 1H DNP times, which would lead to a non-uniform spin temperature. This contribution is expected to be minor.
The decay of 13C polarization during the 1H DNP build-up interval shown in Fig. 2 occurs when the microwave source is active and the 13C nuclear spin ensemble relaxes towards the spin temperature it would have achieved in the case of direct 13C DNP, i.e., no CP. This 13C polarization decay is a combination of three factors: (i) the microwaves are active and hence polarization is diminishing towards the low DNP equilibrium of the 13C nuclear spins with TEMPOL as the polarizing agent; (ii) the 13C nuclear spins are being actively pulsed, although minimally, every 5 s, which leads to an accumulative loss of 13C NMR signal intensity over many minutes; and (iii) the radical concentration and temperature are in an optimal range for thermal mixing (Guarin et al., 2017), and since the 13C spins are polarized whilst the 1H spins are saturated, the two nuclear pools most likely exchange energy via the electron non-Zeeman reservoir, which influences the time evolution of the 13C magnetization until the 1H spins achieve the same spin temperature. The difference in the 13C polarizations PC at 1H DNP time = 24 min for positive and negative microwave irradiation is associated with the 1H polarization build-ups and the performance efficiency of the multiple-contact CP rf pulses; see the Supplement.
The 13C NMR line shapes of [2-13C]sodium acetate shown in Fig. 3 have features which mainly originate from 13C chemical shift anisotropy (CSA) (max ∼ 1.5 kHz at our magnetic field of 7.05 T) and 1H–13C dipolar couplings (typ. −22.7 kHz) that are affected by possible methyl group rotation. Since the 13C CSA is negligible with respect to the 1H–13C dipolar couplings, it is assumed that the 1H–13C dipolar couplings play the key role in the 13C NMR line shape of [2-13C]sodium acetate. The smaller 13C NMR peak contributions observed near the baseline in Fig. 3a likely correspond to different chemical environments within the sample which are being polarized on different timescales.
The values of , PH and the order of the polynomial fit β presented in Fig. 5 are likely to depend to a small degree on the solvent constituents. In the case of our sample, the glycerol-d8 present in the dDNP glassing matrix yields an approximate 13C concentration of ∼ 410 mM at natural abundance, which is ∼ 14 % of the total 13C spin concentration. Under microwave irradiation, the natural abundance 13C spins of glycerol-d8 will be polarized with their own build-up rate and maximum polarization, and although deuterated glycerol-d8 can also be polarized by 1H–13C CP (Vuichoud et al., 2014). As such, these contributions could impact the 13C NMR peak intensities, which would go some way to explaining why the 13C NMR spectra are not of the same overall profile under positive and negative microwave irradiation at long proton DNP times; see Fig. 3b and c. It is also possible that the dipolar couplings and CSA interactions manifest differently under positive and negative microwave irradiation, and there is a preferred energy state for coupling to positive and negative 1H polarizations PH leading to non-identical 13C NMR spectra.
The NMR spectra presented in Fig. 3 were acquired for the cases of high 13C SNRs, the largest of which is ca. 1244. In the event that CP cannot be (efficiently) implemented, and the acquired 13C NMR signal is weak, we anticipate that the method is robust with respect to a few kilohertz of line broadening, which can be used to improve the experimental SNR. The value of the 13C NMR peak CoG normalized deviation is, however, likely to be sensitive to changes in phase, and this should therefore be taken into account before comparing experimental results to any calibration curves similar to those presented in Fig. 5. It is also possible that additional phase corrections may help the trend shown in Fig. 5 move closer to a linear fit for values of < 0.02.
The results of this study suggest that other 13C-labelled molecules which might display distinct solid-state 13C NMR spectra, such as [1-13C]sodium formate and other 13CH3 (or 13CH2) group bearing molecular candidates (presence of a strong 1H–13C dipolar coupling), could also be used as indirect 1H polarization meters (polarimeters). To effectively polarize both 1H and 13C nuclear spins, future experiments could use a tailored mixture of radical species, in certain cases. Clearly, at low levels of 1H polarization PH the lower-intensity resonance is unresolved and polluted by the more intense peak, and as such; the presented analysis could be further improved by considering Voigt fits of the complicated 13C NMR spectra, but since there are a number of resonances to consider this route would lead us away from our simple pedagogical approach.
We have demonstrated that 13C NMR line shape polarimetry of [2-13C]sodium acetate can be implemented to indirectly infer the 1H polarization of the 13CH3 group nuclear spins and potentially the whole sample if the constituents of which are sufficiently homogeneously mixed. An easy to implement protocol based on the normalized deviation of the centre of gravity of the 13C NMR peaks was employed and a simple relationship with 1H polarization was found. This approach is complementary to traditional methods of measuring 1H polarization, in suitable circumstances, and could be useful in situations where measurements of 1H polarizations prove difficult, e.g., due to radiation damping (Mao and Ye, 1997; Krishnan and Murali, 2013), which can also likely impact the experimental data and order of the polynomial fit shown in Fig. 5. Other appropriate cases for potential implementation include the following: (i) the lack of a 1H rf coil, (ii) the presence of large background signals and (iii) the absence of a thermal equilibrium spectrum. The approach presented here works well for traditional dDNP-compatible sample formulations, but future studies employing fully deuterated dDNP solutions could provide 13C NMR line shapes with more distinct features.
Experimental data are available upon request from the corresponding author.
The supplement related to this article is available online at: https://doi.org/10.5194/mr-2-643-2021-supplement.
SJE conceived the idea, performed experiments, processed the data and wrote the article, QS assisted with experiments and data processing and provided useful advice, and SJ provided informative guidance, supportive feedback and contributed to the article.
The authors declare that they have no conflict of interest.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue “Geoffrey Bodenhausen Festschrift”. It is not associated with a conference.
The authors gratefully acknowledge Bruker Biospin for providing the prototype dDNP polarizer and particularly Dmitry Eshchenko, Roberto Melzi, Marc Rossire, Marco Sacher and James Kempf for scientific and technical support. The authors additionally acknowledge Catherine Jose and Christophe Pages for use of the ISA Prototype Service, Stéphane Martinez of the UCBL mechanical workshop for machining parts of the experimental apparatus, Aurélien Bornet for preliminary work, Christian Bengs for useful discussion, and the reviewers for their comments which led to the improvement of this article.
This research has been supported by ENS-Lyon, the French CNRS, Lyon 1 University and the European Research Council under the European Union's Horizon 2020 research and innovation programme (ERC grant agreements no. 714519/HP4all and Marie Skłodowska-Curie grant agreement no. 766402/ZULF).
This paper was edited by Daniel Abergel and reviewed by Andrea Capozzi and one anonymous referee.
Abragam, A., and Goldman, M.: Principles of dynamic nuclear polarisation, Rep. Prog. Phys., 41, 395–467, https://doi.org/10.1088/0034-4885/41/3/002, 1978.
Aghelnejad, B., Marhabaie, S., Baudin, M., Bodenhausen, G., and Carnevale, D.: Spin Thermometry: A Straightforward Measure of Millikelvin Deuterium Spin Temperatures Achieved by Dynamic Nuclear Polarization, J. Phys. Chem. Lett., 11, 3219–3225, https://doi.org/10.1021/acs.jpclett.0c00713, 2020.
Ardenkjær-Larsen, J. H., Fridlund, B., Gram, A., Hansson, G., Hansson, L., Lerche, M. H., Servin, R., Thaning, M., and Golman, K.: Increase in signal-to-noise ratio of > 10 000 times in liquid-state NMR, P. Natl. Acad. Sci. USA, 100, 10158–10163, https://doi.org/10.1073/pnas.1733835100, 2003.
Bornet, A., Milani, J., Vuichoud, B., Perez Linde, A. J., Bodenhausen, G., and Jannin, S.: Microwave frequency modulation to enhance Dissolution Dynamic Nuclear Polarization, Chem. Phys. Lett., 602, 63–67, https://doi.org/10.1016/j.cplett.2014.04.013, 2014.
Bornet, A., Pinon, A. C., Jhajharia, A., Baudin, M., Ji, X., Emsley, L., Bodenhausen, G., Ardenkjær-Larsen, J. H., and Jannin, S.: Microwave-gated dynamic nuclear polarization, Phys. Chem. Chem. Phys., 18, 30530–30535, https://doi.org/10.1039/C6CP05587G, 2016.
Chen, H.-Y., Aggarwal, R., Bok, R. A., Ohliger, M. A., Zhu, Z., Lee, P., Goodman, J. W., van Criekinge, M., Carvajal, L., Slater, J. B., Larson, P. E. Z., Small, E. J., Kurhanewicz, J., and Vigeron, D. B.: Hyperpolarized 13C-pyruvate MRI detects real-time metabolic flux in prostate cancer metastases to bone and liver: a clinical feasibility study, Prostate Cancer P. D., 23, 269–276, https://doi.org/10.1038/s41391-019-0180-z, 2020.
Cheng, T., Capozzi, A., Takado, Y., Balzan, R., and Comment, A.: Over 35 % liquid-state 13C polarization obtained via dissolution dynamic nuclear polarization at 7 T and 1 K using ubiquitous nitroxyl radicals, Phys. Chem. Chem. Phys, 15, 20819–20822, https://doi.org/10.1039/C3CP53022A, 2013.
Dale, M. W. and Wedge, C. J.: Optically generated hyperpolarization for sensitivity enhancement in solution-state NMR spectroscopy, Chem. Commun., 52, 13221–13224, https://doi.org/10.1039/C6CC06651H, 2016.
Dumez, J.-N., Vuichoud, B., Mammoli, D., Bornet, A., Pinon, A. C., Stevanato, G., Meier, B., Bodenhausen, G., Jannin, S., and Levitt, M. H.: Dynamic Nuclear Polarization of Long-Lived Nuclear Spin States in Methyl Groups, J. Phys. Chem. Lett., 8, 3549–3555, https://doi.org/10.1021/acs.jpclett.7b01512, 2017.
Elliott, S. J., Meier, B., Vuichoud, B., Stevanato, G., Brown, L. J., Alonso-Valesueiro, J., Emsley, L., Jannin, S., and Levitt, M. H.: Hyperpolarized long-lived nuclear spin states in monodeuterated methyl groups, Phys. Chem. Chem. Phys., 20, 9755–9759, https://doi.org/10.1039/C8CP00253C, 2018.
Elliott, S. J., Ceillier, M., Cala, O., Stern, Q., Cousin, S. F., El Daraï, T., and Jannin, S.: in preparation, 2021a.
Elliott, S. J., Stern, Q., Ceillier, M., El Daraï, T., Cousin, S. F., Cala, O., and Jannin, S.: Practical Dissolution Dynamic Nuclear Polarization, Prog. Nucl. Magn. Reson. Spectrosc., 126–127, 59–100, https://doi.org/10.1016/j.pnmrs.2021.04.002, 2021b.
Gallagher, F. A., Woitek, R., McLean, M. A., Gill, A. B., Garcia, R. M., Provenzano, E., Reimer, F., Kaggie, J., Chhabra, A., Ursprung, S., Grist, J. T., Daniels, C. J., Zaccagna, F., Laurent, M.-C., Locke, M., Hilborne, S., Frary, A., Torheim, T., Boursnell, C., Schiller, A., Patterson, I., Slough, R., Carmo, B., Kane, J., Biggs, H., Harrison, E., Deen, S. S., Patterson, A., Lanz, T., Kingsbury, Z., Ross, M., Basu, B., Baird, R., Lomas, D. J., Sala, E., Watson, J., Rueda, O. M., Chin, S.-P., Wilkinson, I. B., Graves, M. J., Abraham, J. E., Gilbert, F. J., Caidas, C., and Brindle, K. M.: Imaging breast cancer using hyperpolarized carbon-13 MRI, P. Natl. Acad. Sci. USA, 117, 2092–2098, https://doi.org/10.1073/pnas.1913841117, 2020.
Guarin, D., Marhabaie, S., Rosso, A., Abergel, D., Bodenhausen, G., Ivanov, K. L., and Kurzbach, D.: Characterizing Thermal Mixing Dynamic Nuclear Polarization via Cross-Talk between Spin Reservoirs, J. Phys. Chem. Lett., 8, 5531–5536, https://doi.org/10.1021/acs.jpclett.7b02233, 2017.
Hirsch, M. L., Kalechofsky, N., Belzer, A., Rosay, M., and Kempf, J. G.: Brute-Force Hyperpolarization for NMR and MRI, J. Am. Chem. Soc., 137, 8428–8434, https://doi.org/10.1021/jacs.5b01252, 2015.
Kouřil, K., Kouřilová, H., Bartram, S., Levitt, M. H., and Meier, B.: Scalable dissolution-dynamic nuclear polarization with rapid transfer of a polarized solid, Nat. Commun., 10, 1733, https://doi.org/10.1038/s41467-019-09726-5, 2019.
Krishnan, V. V. and Murali, N.: Radiation damping in modern NMR experiments: Progress and challenges, Prog. Nucl. Magn. Reson. Spectrosc., 68, 41–57, https://doi.org/10.1016/j.pnmrs.2012.06.001, 2013.
Kuhns, P., Gonen, O., and Waugh, J. S.: Proton spin-spin and spin-lattice relaxation in CaSO4 ⋅ xH2O below 1 K, J. Magn. Reson., 82, 231–237, https://doi.org/10.1016/0022-2364(89)90027-9, 1989.
Kundu, K., Mentink-Vigier, F., Feintuch, A., and Vega, S.: DNP mechanisms, eMagRes, 8, 295–338, 2019.
Kuzma, N. N., Håkansson, P., Pourfathi, M., Ghosh, R. K., Kara, H., Kadlecek, S. K., Pileio, G., Levitt, M. H., and Rizi, R. R.: Lineshape-based polarimetry of dynamically-polarized 15N2O in solid-state mixtures, J. Magn. Reson., 234, 90–94, https://doi.org/10.1016/j.jmr.2013.06.008, 2013.
Latanowicz, L.: NMR relaxation study of methyl groups in solids from low to high temperatures, Concept Magn. Reson. A, 27A, 38–53, https://doi.org/10.1002/cmr.a.20040, 2005.
Mammoli, D., Salvi, N., Milani, J., Buratto, R., Bornet, A., Sehgal, A. A., Canet, E., Pelupessy, P., Carnevale, D., Jannin, S., and Bodenhausen, G.: Challenges in preparing, preserving and detecting para-water in bulk: overcoming proton exchange and other hurdles, Phys. Chem. Chem. Phys., 17, 26819–26827, https://doi.org/10.1039/C5CP03350K, 2015.
Mao, X. A. and Ye, C. H.: Understanding radiation damping in a simple way, Concepts Magn. Reson. A, 9, 173–187, https://doi.org/10.1002/(SICI)1099-0534(1997)9:3<173::AID-CMR4>3.0.CO;2-W, 1997.
Marohn, J. A., Carson, P. J., Hwang, J. Y., Miller, M. A., Shykind, D. N., and Weitekamp, D. P.: Optical Larmor beat detection of high-resolution nuclear magnetic resonance in a semiconductor heterostructure, Phys. Rev. Lett., 75, 1364–1367, https://doi.org/10.1103/PhysRevLett.75.1364, 1995.
Meier, B.: Quantum-rotor-induced polarization, Magn. Reson. Chem., 56, 610–618, https://doi.org/10.1002/mrc.4725, 2018.
Meier, B., Dumez, J.-N., Stevanato, G., Hill-Cousins, J. T., Roy, S. S., Håkansson, P., Mamone, S., Brown, R. C. D., Pileio, G., and Levitt, M. H.: Long-Lived Nuclear Spin States in Methyl Groups and Quantum-Rotor-Induced Polarization, J. Am. Chem. Soc., 135, 18746–18749, https://doi.org/10.1021/ja410432f, 2013.
Nelson, S. J., Kurhanewicz, J., Vigneron, D. B., Larson, P. E. Z., Harzstark, A. L., Ferrone, M., van Criekinge, M., Chang, J. W., Bok, R., Park, I., Reed, G., Carvajal, L., Small, E. J., Munster, P., Weinberg, V. K., Ardenkjær-Larsen, J. H., Chen, A. P., Hurd, R. E., Odegardstuen, L.-I., Robb, F. J., Tropp, J., and Murray, J. A.: Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate, Sci. Trans. Med., 5, 198ra108, https://doi.org/10.1126/scitranslmed.3006070, 2013.
Roy, S. S., Dumez, J.-N., Stevanato, G., Meier, B., Hill-Cousins, J. T., Brown, R. C. D., Pileio, G., and Levitt, M. H.: Enhancement of quantum rotor NMR signals by frequency-selective pulses, J. Magn. Reson., 250, 25–28, https://doi.org/10.1016/j.jmr.2014.11.004, 2015.
Vuichoud, B., Milani, J., Bornet, A., Melzi, R., Jannin, S., and Bodenhausen, G.: Hyperpolarization of Deuterated Metabolites via Remote Cross-Polarization and Dissolution Dynamic Nuclear Polarization, J. Phys. Chem. B, 118, 1411–1415, https://doi.org/10.1021/jp4118776, 2014.
Waugh, J. S., Gonen, O., and Kuhns, P.: Fourier transform NMR at low temperatures, J. Chem. Phys., 86, 3816–3818, https://doi.org/10.1063/1.451940, 1987.
Willmering, M. M., Ma, Z. L., Jenkins, M. A., Conley, J. F., and Hayes, S. E.: Enhanced NMR with Optical Pumping Yields 75As Signals Selectively from a Buried GaAs Interface, J. Am. Chem. Soc., 139, 3930–3933, https://doi.org/10.1021/jacs.6b08970, 2017.




